
THE “WILL THIS TRIAL CHANGE MY PRACTICE?” SESSION AT EUROPCR 2019
The aim of this article is to capture the session at EuroPCR 2019, communicate the analysis of the trialists, and report the views expressed in the interactive discussion.
Introduction to the session
The last 17 years represent an unparalleled era of technological design and device iterations for valve intervention. Transcatheter aortic valve implantation (TAVI) has been explored in randomised controlled trials (RCTs) in an inverse fashion when compared with coronary angioplasty - starting with extreme- and high-risk patients, and progressively moving towards low-risk patients (Figure 1).
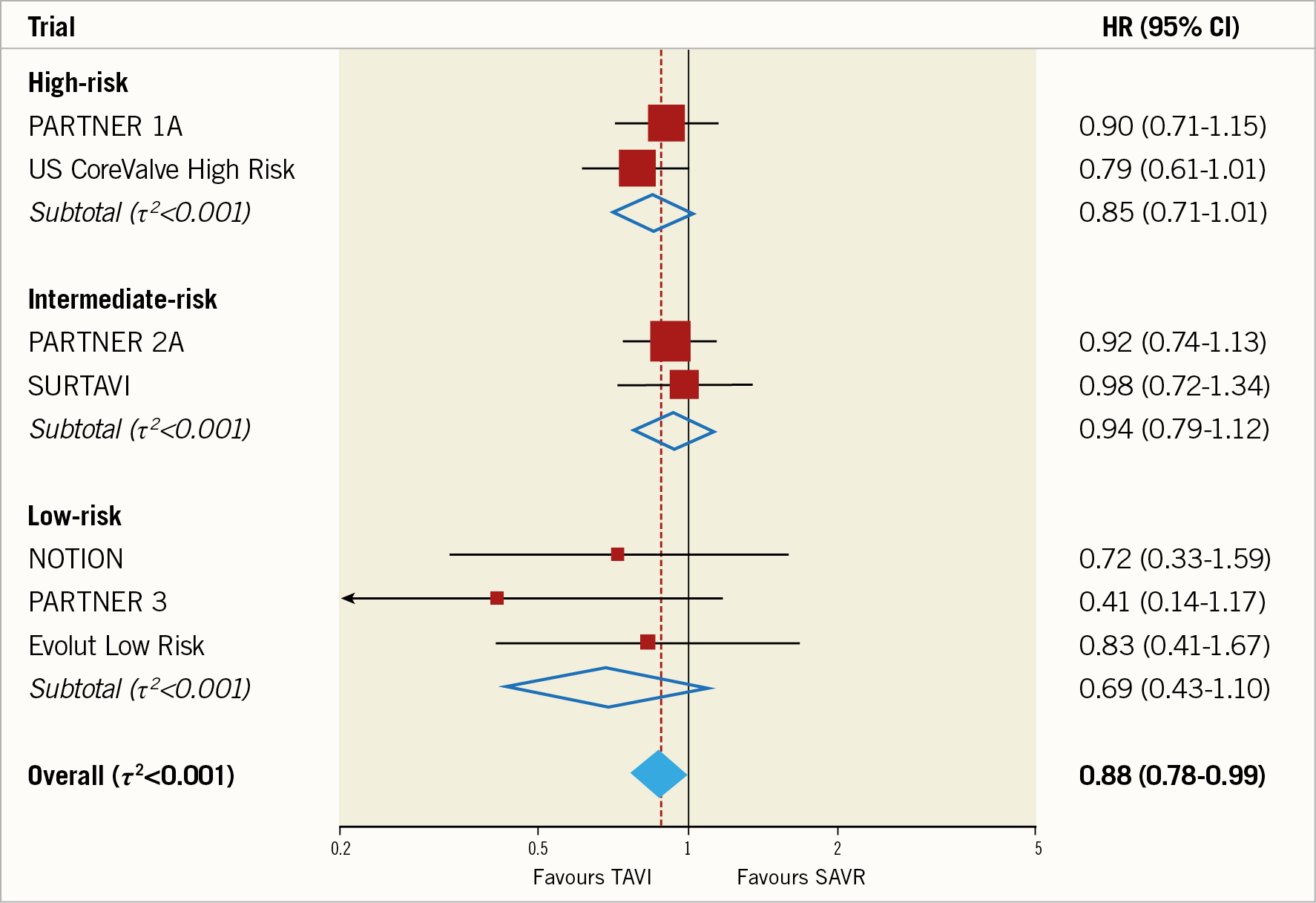
Figure 1. Meta-analysis for the primary outcome all-cause mortality comparing TAVI versus SAVR up to two-year follow-up stratified by baseline surgical risk at study level (seven randomised trials, n=8,020 patients). Reproduced with permission from Siontis et al7.
The presentation of the PARTNER 3 Trial1 at the American College of Cardiology Scientific Session just two months before was commented on by Eugene Braunwald as “an historic moment”. In the light of this landmark trial, TAVI in low-risk patients was discussed at EuroPCR 2019 in a “Will this trial change my practice?” session. The chairs, Thomas Modine and Stephan Windecker, directed the session, inviting frequent audience participation and polling on current and intended future practice based on this study. Azeem Latib presented the available literature prior to the release of outcome data in low-risk cohorts.
THE CASE PRESENTATION
The principal investigator, Professor Martin Leon, presented a case from the trial – an 80-year-old female who was still in employment with only mild hypertension as a comorbidity. Her Society of Thoracic Surgeons (STS) score was 1.5%, an electrocardiogram (ECG) demonstrated baseline right bundle branch morphology, and her anatomy was suitable for TAVI. She was randomised to transcatheter intervention, which was carried out under conscious sedation and rapid right ventricular pacing, and a SAPIEN 3 (S3) 23 mm prosthesis (Edwards Lifesciences, Irvine, CA, USA) was implanted intentionally high. There was an excellent echocardiographic outcome and no change in baseline ECG, and she was discharged on day 1 with significantly improved symptoms reported at 30-day follow-up.
In this RCT of 1,000 patients, those undergoing TAVI spent less time undergoing the intervention (TAVI procedural times were under one hour) and less time in hospital (p<0.001), and were more frequently discharged to their own home (p<0.001). They had reduced rates of periprocedural bleeding, new-onset atrial fibrillation at 30 days (5% vs 39.5%, p<0.001), stroke at 30 days in the absence of cerebral embolic protection devices (0.6% vs 2.4%, p=0.02), composite endpoint of all-cause mortality, stroke and rehospitalisation at 12 months (8.5% vs 15.1%, p=0.001), and disabling stroke and all-cause mortality at 12 months (1.0% vs 2.9%, hazard ratio 0.34, 95% CI: 0.12 to 0.97) (Figure 2). No difference was found in the incidence of new permanent pacemaker insertion and major vascular complications. Professor M. Leon summarised by saying that, based on these findings up to 12 months, TAVI should be considered as the preferred therapy in low surgical risk aortic stenosis patients with favourable aortic valve dimensions and iliofemoral access.
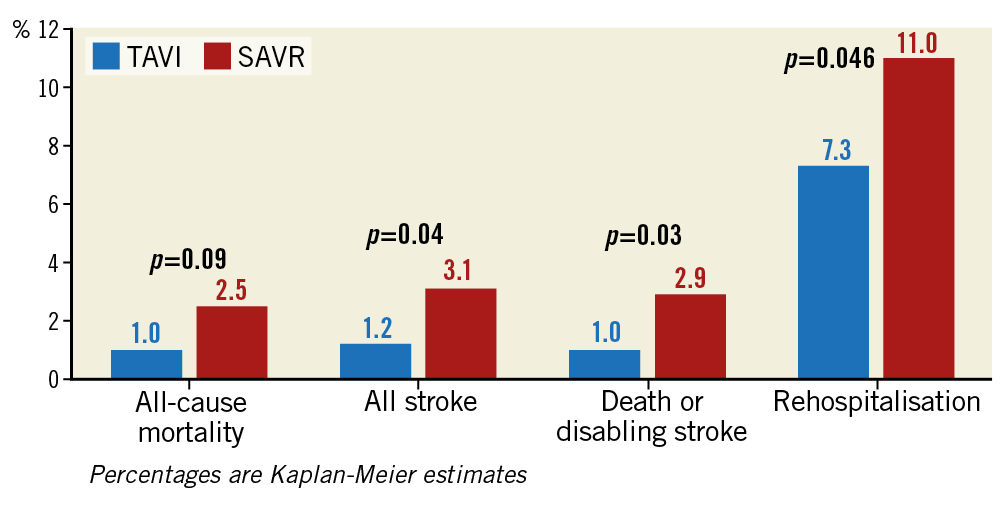
Figure 2. Clinical endpoints from the PARTNER 3 Trial.
BACKGROUND: WHAT WAS KNOWN BEFORE THE TRIAL?
The 2017 ESC/EACTS guidelines2 recommend TAVI in patients who are not suitable for surgical aortic valve replacement (SAVR), TAVI or SAVR for those at increased surgical risk (STS score ≥4%) as assessed by the Heart Team, and SAVR in patients at low surgical risk (STS score <4% and no other risk factors not included in the risk scores, such as frailty, porcelain aorta, or sequelae of chest radiation), Class 1, level of evidence B. The 2017 AHA/ACC guidelines3 similarly recommend TAVI in patients who are not suitable for SAVR, and SAVR or TAVI in high-risk surgical candidates, Class I, level A, and state that TAVI is a reasonable alternative to SAVR in intermediate-risk patients (Class IIa, B-R). Again, severe symptomatic aortic stenosis in a patient at low surgical risk is currently a Class I indication for SAVR.
Surgeons typically quote a 1% risk of serious harm for low-risk patients undergoing SAVR. The challenge was therefore to see whether TAVI could compete with this. Professor M. Leon commented that this trial allowed comparison of the two interventions side by side.
TRIAL ANALYSIS: SUMMARY OF THE TRIALISTS’ CRITICAL REVIEW
The trial design was presented by Professor Peter Jüni, a clinical epidemiologist and trialist. This randomised multicentre, non-inferiority trial which ran from March 2016 to October 2017 had 1:1 randomisation of 1,000 patients across 71 sites with randomisation stratified according to recruiting centres and blocked with fixed block sizes of four. Professor P. Jüni explained that fixed block sizes in an open trial in the presence of stratified randomisation by site make the trial somewhat vulnerable to selection bias caused by investigators potentially subverting concealment of allocation if they were aware of the fixed block size, and therefore theoretically able to predict future treatment allocation in every fourth patient. Fixed block sizes should therefore not be used in future open TAVI trials, or at the very least not be communicated in the trial protocols. Eligibility was based on severe calcific aortic stenosis, STS predicted risk of mortality (PROM) score <4%, site Heart Team and trial committee approval, and anatomical suitability for TAVI using an S3 prosthesis. Exclusion criteria included clinical frailty, bicuspid anatomy and other anatomic features that increase the risk of complications associated with TAVI/SAVR. The lowest participant’s age was 57 years old, and almost 10% of patients were under 65 years old.
The primary outcome was a composite of all-cause mortality, stroke or rehospitalisation at 12 months; neurological examinations were carried out at baseline and at 30 days. The trial was powered for non-inferiority on the primary outcome at a margin of 6.0%, assuming an event rate of 14.6% in the TAVI group and 16.6% in the SAVR group. Assuming a difference in event rates between groups is unusual for a non-inferiority trial and may be criticised by some as being conceptionally inconsistent; however, P. Jüni emphasised that this does not invalidate the sample size consideration. The cohorts had similar surgical risk (STS score 1.9% in each) and baseline characteristics. Patient preference resulted in 35 patients withdrawing from the cohort randomised to SAVR.
SESSION DISCUSSION
The previous three PARTNER trials4,5,6 have >5,000 citations. This RCT took place in a period of TAVI procedural evolution in which the landscape was changing with technology, patient risk profile and increasing operator experience. This increased operator familiarity and confidence in TAVI was evident during polling and audience opinion screening.
Geographical disparity was significant; some centres are already treating low-risk patients with TAVI, and in many centres surgeons are centrally involved in the TAVI decision making and procedures. There are, however, reimbursement obstacles before offering TAVI outside the current guidelines in most parts of the world, predominantly in the USA. On the whole, most attendees still valued the Heart Team in making decisions for patients with aortic stenosis.
In some regions, patients aged >80 years are referred for TAVI without surgical consult, but there is currently limited capacity to offer TAVI at a younger age. Patient preference almost always favours TAVI and, with overwhelmingly supportive data from a series of trials, there is an increasing need to expand the indication. The age threshold for TAVI in low-risk patients was debated: in view of an average life expectancy of 80 years for men, and 84 years for women, 65-70 years old was felt to be a reasonable cut-off since this is the current age range expected to undergo implantation of a surgical bioprosthesis. The concern regarding valve durability is important and correlates positively with the patient’s age at implant (rather than the life expectancy of a low-risk 75-year-old). There is no reported difference between TAVI and SAVR prosthesis durability up to 10 years; however, more long-term data from randomised trials are needed to provide reassurance. As we move towards transcatheter intervention in younger patients, clinicians should have a transparent and frank upfront discussion about future options in case of degeneration (Figure 3).
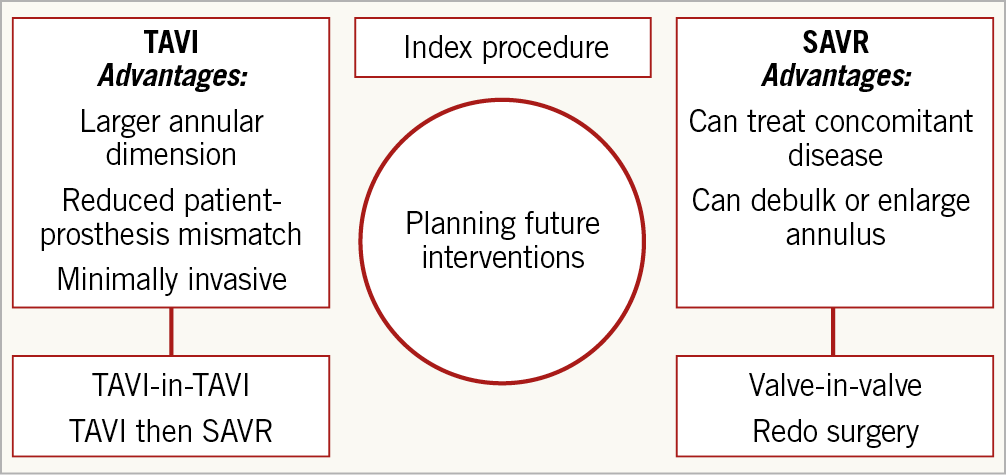
Figure 3. Planning future interventions
The importance of preoperative ECG-gated computed tomography from the aortic arch to the femoral arteries was emphasised as a necessity for procedural planning. This provides important information on annular dimensions for valve sizing, burden of calcification of the aorta, annulus and left ventricular outflow tract, distance of the coronary arteries to the annulus, and access route sizing. Particularly in the setting of pre-existing right bundle branch block morphology, implant depth and valve choice can be contributory to the need for permanent pacing and the choice of TAVI versus SAVR – this argument is more poignant in younger patients.
The appeal of TAVI is in its simplicity and reproducibility of results. In this trial, concomitant procedures were carried out in 7.9% versus 26.4% of the TAVI and SAVR cohorts, respectively. Professor M. Leon suggested that surgeons may be doing too many additional procedures without clinical benefit, often decided in the operating theatre. Of note, subgroup analysis of TAVI and SAVR without concomitant procedures also demonstrated higher incidence in the primary composite endpoint with SAVR.
Over the past 12 years, the PARTNER randomised trials have proved that the relative benefit of TAVI compared with surgery is independent of surgical risk profiles and that the choice for each patient should be based on a shared decision-making process where patient preferences should be respected. Surgeons must engage with TAVI – integrative working in centres of excellence will enable the best individual decision making for patients and, despite an expected fall in SAVR numbers, surgical training should not be prohibitive. Despite being in widespread use, surgical risk scores are not appropriate for TAVI.
Conclusion
There has been a systematic decrease in event rates with increased operator experience, device improvement and patient selection for TAVI. Transfemoral TAVI has been validated by robust randomised clinical data demonstrating superiority over SAVR in low- and intermediate-risk patients and will become the default therapy for most surgical patients requiring implantation of a bioprosthesis. Ongoing research into valve durability remains relevant.
Conflict of interest statement
A. Latib reports consulting for Medtronic, Abbott Vascular and Edwards Lifesciences. M. Leon reports research grants from Edwards Lifesciences, Boston Scientific and Medtronic, and being a member of the structural advisory board of Gore. P. Jüni reports serving as an unpaid member of the steering group of cardiovascular trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical, and The Medicines Company, and has received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and The Medicines Company, and honoraria to the institution for participation in advisory boards from Amgen, but has not received personal payments from any pharmaceutical company or device manufacturer. S. Windecker reports research and educational grants to the institution from Amgen, Abbott, BMS, Bayer, Boston Scientific, Biotronik, CLS Behring, Edwards Lifesciences, Medtronic, Polares and Sinomed. The other authors have no conflicts of interest to declare.

