
The “Will this trial change my practice?” sessions at PCR
The aim of the article is to capture this session at EuroPCR 2019, communicate the analysis of the trialists, and report the views expressed in the interactive discussion.
Introduction to the session
The session took the following format: the Chairperson was S. Windecker, with H. Treede as Co-chairperson and C. O’Sullivan as Participant. “The Introduction - the trial headlines” was presented by S. Windecker. A “How should I treat?” case was presented by M. Reardon; “What was known before Evolut Low Risk” was presented by L . Søndergaard; the “Trialists’ review – methods, results, conclusion” was presented by P Jüni, and “Case conclusion – how does this apply to my practice?” by M. Reardon. The “Session evaluation and key lessons” was led by H. Treede.
Transcatheter aortic valve implantation (TAVI) was developed as a less invasive alternative to surgical aortic valve replacement (SAVR) in symptomatic patients with severe aortic stenosis1. In contrast to the clinical introduction of percutaneous coronary intervention (PCI) starting in low-risk patients with symptomatic coronary artery disease, TAVI was introduced in inoperable and high-risk symptomatic patients with severe aortic stenosis with progressively lower-risk groups being tested in randomised trials against surgery2,3,4,5,6,7,8,9. For the self-expanding valve, TAVI was superior to surgery in high-risk patients for the primary endpoint of all-cause mortality at one and at two years5,6. TAVI has since been shown to be non-inferior to SAVR at two years in intermediate-risk patients for a primary endpoint of all-cause mortality or disabling stroke7. These encouraging findings led to randomised trials in low-risk patients comparing TAVI against SAVR.
The Evolut Low Risk (LR) randomised controlled trial (RCT) was presented at the 2019 American College of Cardiology Scientific Session along with the Placement of Aortic Transcatheter Valves 3 (PARTNER 3) RCT for the balloon-expandable valve and published simultaneously in the New England Journal of Medicine10,11. The Evolut LR trial demonstrated non-inferiority of TAVI compared to SAVR with regard to combined all-cause mortality and disabling stroke at two years, whereas TAVI was superior to SAVR in PARTNER-3 using all-cause mortality, disabling stroke and re-hospitalisation at one year as primary endpoint. Recognising the potential for a paradigm shift in the treatment of aortic stenosis, EuroPCR held a session looking at these two trials asking the question “Will this trial change my practice?”. This report summarises the Evolut LR trial and its potential impact on clinical practice10.
THE CASE PRESENTATION
Principal investigator, Michael Reardon, presented a representative case from the Evolut LR study involving a 77-year-old female with New York Heart Association Class III symptoms, mean aortic valve gradient of 44 mmHg, an aortic valve area of 0.5 cm2 and normal left ventricular systolic function. The Society of Thoracic Surgeons (STS) score was 1.8% and computed tomography measurements revealed a 69 mm annular perimeter (derived diameter 21.9 mm). Right femoral access was used with a 20 Fr DrySeal sheath (W.L. Gore & Associates, Inc., Flagstaff, AZ, USA) and the left femoral artery was used to place the pigtail catheter. A temporary venous pacemaker was inserted via the right internal jugular vein. The native aortic valve was predilated with a 20 mm TRUE® balloon (Bard Peripheral Vascular, Inc., Tempe, AZ, USA). A 26 mm Evolut™ PRO valve (Medtronic, Minneapolis, MN, USA) was implanted under general anaesthesia and there was no paravalvular leak. The right femoral access site was closed with one ProGlide® suture (Abbott Vascular, Santa Clara, CA, USA). There was no bundle branch block, no stroke, no acute kidney injury and there were no bleeding complications. The patient was discharged on day two.
This case was consistent with the TAVI cases in the Evolut LR trial where the mean age was 73.6 years and mean STS score was 1.9%. In the Evolut LR trial, patients spent less time in the procedure room, less time in the intensive care unit, less time in the hospital and were more often discharged directly to home. Consistent with data derived from previous RCTs, TAVI was associated with less acute kidney injury, atrial fibrillation and bleeding with transfusions than SAVR. TAVI also had larger effective orifice area, lower transvalvular pressure gradient, and less patient-prosthesis mismatch (PPM) compared to SAVR as seen in all self-expanding RCTs.
BACKGROUND: WHAT WAS KNOWN BEFORE THE TRIAL
Lars Søndergaard reviewed what was known before the low-risk TAVI trials. The Nordic Aortic Valve Intervention (NOTION) trial was a prospective, multicentre, non-blinded, RCT comparing SAVR versus TAVI in patients ≥70 years with severe symptomatic aortic stenosis between December 2009 and April 201312. The mean STS scores in the transcatheter (2.9±1.6%) and surgical (3.1±1.7%) cohorts were low, but the mean age remained relatively high (79.2±4.9 years versus 79.0±4.7 years, respectively). At five years, there was no significant difference in the primary endpoint of the composite rate of all-cause mortality, stroke or myocardial infarction (39.2% vs 35.8%, p=0.78)13. As compared with SAVR, patients undergoing TAVI had a significantly lower rate of structural valve deterioration at six-year follow-up (4.8% vs 24.0%, p<0.001), although bioprosthetic valve failure rates were similar between the groups (7.5% vs 6.7%, p=0.89)14. Patients receiving TAVI had significantly higher rates of permanent pacemaker implantation (43.7% vs 8.7%, p<0.001), and those receiving a permanent pacemaker had a trend towards higher rates of all-cause mortality at five years as compared with patients who did not receive a permanent pacemaker (38.2% vs 21.7%, p=0.07)13. Nevertheless, caution was advised when interpreting these findings because this was a post hoc analysis.
As TAVI shifts to younger patients, there will be a higher prevalence of bicuspid aortic valve disease, which is associated with anatomical challenges such as heterogeneous cusp and sinus morphology, heavy and asymmetric calcifications and long commissural distance in addition to associated aortopathy, coarctation of the aorta and aortic root angulation (horizontal aorta)15. It is important to note that bicuspid aortic valves were excluded from all RCTs performed to date comparing TAVI with SAVR.
The mean age of patients in the Evolut LR and the PARTNER 3 trials was 74 years, with the youngest patient included being under 60 years of age. The 2017 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guideline document states that TAVI should be considered at 75 years, which may be reconsidered in view of the low-risk RCTs16. The durability of transcatheter heart valves (THVs) was discussed, and it was mentioned that the durability of THVs is similar to surgical bioprostheses at six years, as assessed in RCTs14.
The complex relationship between THVs and the coronary arteries in TAVI is an important consideration. For TAVI in native aortic valves, the stent frame of the THV may overlay the ostia of the coronary arteries, rendering access to the coronary arteries potentially challenging. Coronary access is potentially more difficult after valve-in-valve (ViV) TAVI.
TRIAL ANALYSIS: SUMMARY OF THE TRIALISTS’ CRITICAL REVIEW
Peter Jüni reviewed the Evolut LR trial design and outcomes. The Evolut LR trial was a randomised non-inferiority trial in which TAVI with a self-expanding supra-annular bioprosthesis was compared with SAVR in patients who had severe aortic stenosis and were at low surgical risk10. Randomisation was adequately concealed using an electronic randomisation system. It was stratified by site and by the need for coronary revascularisation and blocked using variable block sizes. The independent clinical events committee was blinded when feasible; however, for some endpoints knowledge of treatment allocation was inherent in the endpoint assessment.
In this low-risk population, event rates are expected to be lower than in the higher-risk trials. It was originally assumed that 1,200 patients would yield more than 85% power to detect non-inferiority at an alpha of 0.05 on the basis of a 15% incidence of death or disabling stroke at 24 months in both groups. A total of 1,468 patients were ultimately enrolled to permit completion of a randomised substudy and to meet Japanese regulatory requirements. The trialists chose to stay with the objective endpoints of all-cause mortality or disabling stroke to remain consistent with the Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) intermediate-risk trial7. An adaptive Bayesian design allowed an appropriate early look 12 months after the 850th patient underwent the study procedure.
As the primary analysis was a non-inferiority analysis, it was performed in the as-treated population defined as the population of 1,403 randomly assigned patients who underwent an attempted procedure. For patients who did not complete 24 months of follow-up, the 24-month outcome was imputed according to a pre-specified statistical model based on the patients’ last known clinical status. The early look showed that the posterior probability of non-inferiority exceeded the pre-specified threshold of 0.972 and allowed data analysis to proceed. The two-year estimates of all-cause mortality or disabling stroke were 6.7% and 5.3% for SAVR and TAVI, respectively, establishing non-inferiority at two years (posterior probability of non-inferiority >0.999), albeit at a much lower event rate than originally assumed (Figure 1). The conclusion of the Evolut LR trial was that the Evolut R was non-inferior to TAVI10.
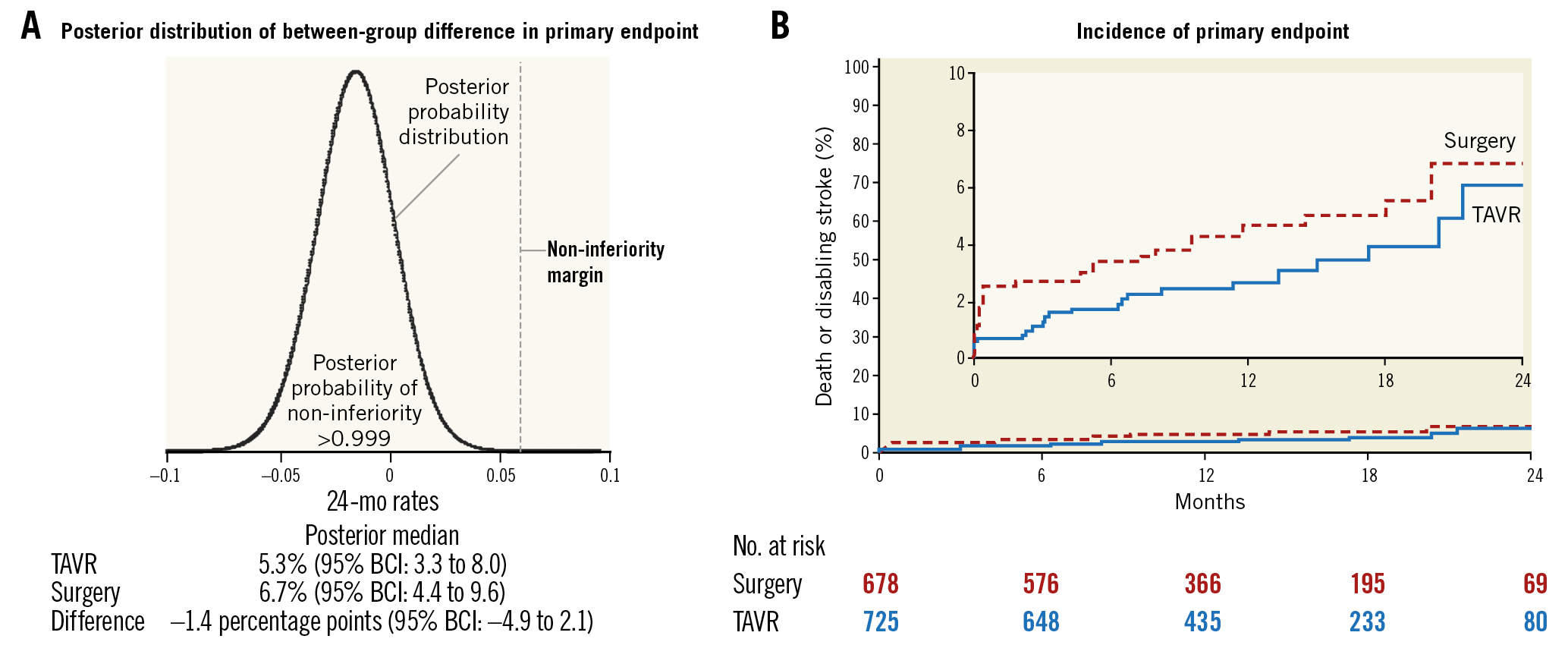
Figure 1. Posterior distribution and time-to-event curves for the primary endpoint. The posterior distribution for the difference between the treatment groups in the incidence of death from any cause or disabling stroke at 24 months (the primary endpoint), shown in panel A, confirmed that the non-inferiority criterion for the primary endpoint was met. B) Kaplan-Meier time-to-event curves for the primary endpoint. The inset shows the same data on an enlarged y-axis. BCI: Bayesian credible interval; TAVR: transcatheter aortic valve replacement. Permission has been obtained to reprint this Figure from the Massachusetts Medical Society.
THE PRACTITIONERS’ VIEW
Michael Reardon then presented how the low-risk trials have changed his practice from a cardiac surgeon’s point of view. He noted that the Evolut LR trial showed non-inferiority for the two-year endpoint of all-cause mortality or disabling stroke10. In the RCTs comparing both therapeutic modalities, SAVR was observed to have superior valvular haemodynamics as compared with TAVI using the balloon-expandable Edwards SAPIEN 3 prosthesis (Edwards Lifesciences, Irvine, CA, USA) in the PARTNER 3 trial11. Conversely, valve haemodynamics were significantly improved with TAVI versus SAVR among TAVI patients treated with the self-expanding Evolut prosthesis in the Evolut LR trial10. When comparing the TAVI groups in Evolut LR with those of PARTNER 3 there was a significantly lower rate of PPM, defined as an indexed effective orifice area ≤0.85 cm2/m2, among Evolut TAVI patients, which was due to the supra-annular design of the Evolut THV. As PPM has been shown to have a negative impact on prognosis, exercise tolerance and re-hospitalisation in patients after surgical bioprosthetic aortic valve replacement, it will be important to evaluate the impact of these differences on long-term clinical outcomes17. Data from the SURTAVI trial demonstrated that, as compared with SAVR, patients undergoing TAVI with the CoreValve® (Medtronic) had significantly better six-minute walk tests as compared with baseline at 30 days (change from baseline 35.4 m vs 14.4 m, p<0.01), one year (change from baseline 37.1 m vs 17.8 m, p<0.01) and two years (change from baseline 26.5 m vs 11.7 m, p=0.04). When PPM was stratified by annular size (i.e., small, medium or large), the rate of moderate or severe PPM increased among patients receiving SAVR bioprosthetic valves in medium or small annuli but this trend was not observed in THVs (Reardon MJ. How Low Risk Has Changed My Practice. Presented at EuroPCR, Paris, France, 21 May, 2019). In conclusion, Michael Reardon said that the low-risk trials have changed his practice and that the number of patients undergoing TAVI will continue to increase. Furthermore, with the recent approval of low-risk TAVI by the Food and Drug Administration in August 2019, patients considered for bioprosthetic SAVR should have TAVI included in the informed consent discussion or else true shared decision making is not taking place. M. Reardon expects the next set of European and US guidelines to reflect these data including low-risk patients for TAVI. The question, therefore, will become when to perform SAVR in the age of TAVI18.
Summary box
ARGUMENTS FOR A CHANGE IN PRACTICE
– Two large multicentre randomised controlled trials have shown equivalent or even superior clinical outcomes among low-risk patients undergoing transcatheter aortic valve implantation (TAVI) as compared with surgical aortic valve replacement (SAVR).
– An updated meta-analysis of randomised controlled trials comparing TAVI with SAVR found that TAVI was associated with a reduction in all-cause mortality and stroke up to two years, irrespective of baseline surgical risk and type of transcatheter heart valve system used.
– TAVI is a less invasive procedure as compared with SAVR.
ARGUMENTS AGAINST A CHANGE IN PRACTICE
– The higher prevalence of bicuspid aortic valves in younger patients, the long-term impact of permanent pacemaker and paravalvular leakage on left ventricular function, coronary access following valve implantation in addition to the durability of the bioprosthetic valves pose unresolved questions that need to be considered in younger patients.
Conflict of interest statement
C.J. O’Sullivan has received consultant fees from Edwards Lifesciences. M.J. Reardon has received research and educational grants to the institution from Medtronic. L. Søndergaard has received consultant fees and institutional research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. H. Treede is a proctor and advisor for Symetis. P. Jüni holds a Tier 1 Canada Research Chair in Clinical Epidemiology of Chronic Diseases, has received research grants to the institution from AstraZeneca, Biotronik, Biosensors International, Eli Lilly, and The Medicines Company, and serves as unpaid member of the steering group of trials funded by AstraZeneca, Biotronik, Biosensors, St. Jude Medical, and The Medicines Company. S. Windecker has received research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Boston Scientific, Biotronik, CSL Behring, Medtronic, Edwards Lifesciences, Polares and Sinomed.

