
Abstract
Transcatheter aortic valve implantation (TAVI) has become the preferred method of treatment for high-risk patients with severe symptomatic aortic stenosis (AS) and is a preferred alternative to surgical valve replacement for intermediate-risk patients. Stroke remains one of the most clinically devastating complications following TAVI. We review the incidence of neurologic injury related to TAVI, proposed definitions for neurologic events and current evidence for neuroprotection and adjunctive pharmacotherapy.
Abbreviations
AS: aortic stenosis
DAPT: dual antiplatelet therapy
OAC: oral anticoagulation
SAPT: single antiplatelet therapy
TAVI: transcatheter aortic valve implantation
VKA: vitamin K antagonist
Introduction
Transcatheter aortic valve implantation (TAVI) has transformed the treatment of patients with severe symptomatic aortic stenosis by virtue of a safe and less morbid minimally invasive approach that is globally more accessible. Global estimates of TAVI procedures are projected to reach 300,000 this year, with a continued 16% annual growth rate, filling a clinical need where many symptomatic patients previously went untreated. TAVI’s rapid adoption is spurred by the rigorous evidence of mortality benefit in inoperable patients and favourable outcomes compared to standard surgical valve replacement (SAVR), leading to guideline adoption and clear patient preference for a less invasive alternative. New indications for low-risk symptomatic patients, for bicuspid valves and patients with severe asymptomatic aortic stenosis are currently under investigation and expected to expand indications for TAVI further in the future.
Stroke risk and implications
Early clinical trials of TAVI brought iatrogenic stroke under scrutiny. High-risk operative candidates randomised to TAVI in the original PARTNER trial had a substantially increased risk of stroke or transient ischaemic attack compared with SAVR at 30 days (5.5% vs. 2.4%)1. The rate was 6.7% among inoperable patients, with the majority of events being disabling strokes2, and over 50% being procedure-related3. In the nine years since the first PARTNER trial enrolment, TAVI has evolved significantly. More recent comparisons generally find a similar or lower stroke risk in TAVI compared with SAVR, probably due to increased operator experience, lower-profile devices and improved designs4,5. However, neurological events continue to affect a substantial proportion of patients, with 30-day stroke rates in the range of 3-6% in recent randomised trials including intermediate-risk patients4,5.
Growing evidence demonstrates that neurological events are in fact underreported in clinical trials. When systematic neurologic evaluation by neurologists and neuroimaging are performed, early stroke rates range from 9% up to 28% after both SAVR and TAVI6-8. Acute stroke detection can be confounded by exposure to anaesthesia, analgesic medications and various post-procedural complications. For example, delirium is now recognised as the presenting symptom of acute stroke in 13-48% of patients and is associated with worse outcomes and higher mortality9. For this reason, delirium should trigger a neurologic assessment for stroke.
Routine neuroimaging studies reveal that ischaemic cerebral infarction caused by showers of cerebral emboli during valve instrumentation and placement affect virtually all patients undergoing TAVI. The total volume of ischaemic brain infarction quantified after TAVI in these imaging studies ranges from 1.5 cm3 to 4.3 cm3 of brain damage which equates to cell death of ≈2 million neurons and ≈1 billion synapses10. These imaging findings have been validated by recent clinical evidence of captured embolic debris in 99% of patients undergoing TAVI with the Montage™ Dual Filter System (Claret Medical, Inc., Santa Rosa, CA, USA), evaluated in the recent CLARET clinical trial. More than 80% of retrieved debris measured 0.15-0.5 cm and <5% of debris measured >1 cm, with histopathology analysis confirming recovery of calcium, thrombus, valve leaflet, arterial wall and catheter material from the TAVI system11.
The clinical consequences of periprocedural cerebral embolisation are generally unpredictable and highly variable, ranging from acutely symptomatic in 9-28% (disabling in up to 4%) of patients to acutely subclinical or “covert” in 72-91%. Large population-based evidence links acutely “subclinical” strokes to significant subsequent cognitive decline, subsequent dementia, and risk of future stroke12,13. Although these longer-term clinical and cognitive consequences remain largely unexplored in the context of iatrogenic cerebral embolisation from cardiac procedures, they are generally considered cumulative effects and should not be dismissed. In contrast, the clinical consequences of periprocedural stroke are devastating. Not only does stroke carry a high risk of mortality, but the severity and permanence of a life-altering disability following stroke is a fate worse than death for most patients14. The facts are that disabling strokes after TAVI carry a threefold to ninefold mortality risk; 40% of survivors have moderate to severe permanent disability leading to dependence; 80% face social isolation and significant financial strain15,16. While patients rated stroke as being 50% to 250% worse than death in a large survey, cardiologists view the death of a patient as being worse than a stroke14. The clinical, social and economic impact of stroke and neurologic injury is likely to be amplified as TAVI therapy expands to younger and healthier patient populations, more vulnerable to the long-term impact of disability from stroke.
Definitions
In 2011, the Valve Academic Research Consortium (VARC), an independent collaboration between European and US academia, specialty societies and regulators from the Food and Drug Administration (FDA), provided a roadmap for standardising the future of TAVI and other aortic valve clinical research17. VARC recommended that only major strokes (defined as modified Rankin score ≥2 at 90 days) be considered as an important safety endpoint for the purposes of clinical trials, while all neurologic events should be reported as adverse events. Since VARC, three updated definition standards for stroke and relevant to TAVI have been formulated (Table 1): Valve Academic Research Consortium (VARC)-218, Standardized Data Collection for Cardiovascular Trials Initiative (SCTI)19, and Neurologic Academic Research Consortium (NeuroARC)20. The SCTI definition for stroke is not specific to TAVI and remains broad and flexible, allowing variable precision depending on the relative importance in a particular trial. Still, the definition is driven by a singular concept in which stroke can be linked to disabling vascular injury. In contrast, VARC-2 and NeuroARC definitions provide a comparatively less malleable framework for a focused application. NeuroARC comprehensively defines the full spectrum of cerebrovascular injury through a combination of well-established symptom-based criteria with sensitive tissue-based findings. NeuroARC defines three major classifications of stroke: “overt (acutely symptomatic) CNS injury (Type 1), covert (acutely asymptomatic) CNS injury (Type 2), and neurologic dysfunction (acutely symptomatic) without CNS injury (Type 3)”. NeuroARC emphasises the central role of imaging, preferably with diffusion-weighted magnetic resonance imaging (DW-MRI), in contemporary tissue-based stroke ascertainment and its systematic incorporation in neuroprotection trials. DW-MRI is significantly more sensitive than computed tomography (CT), it detects ischaemic CNS tissue changes within minutes to days and allows accurate quantification of ischaemic tissue21-23.
The application of stroke definitions with respect to TAVI is an important consideration. VARC-2 definitions have largely been appropriate for assessing the global safety and efficacy of TAVI in comparison to SAVR. However, as TAVI indications grow to include low-risk patients, it will be important to examine stroke through a different, more scrutinising lens, e.g., elucidating the long-term clinical sequelae of procedural silent or covert cerebral infarction. NeuroARC recommendations and standardisations are a step in the right direction in tailoring neurologic evaluation and endpoint selection to a wider range of cardiovascular interventions including neuroprotection.
STROKE AETIOLOGY AND PREVENTION
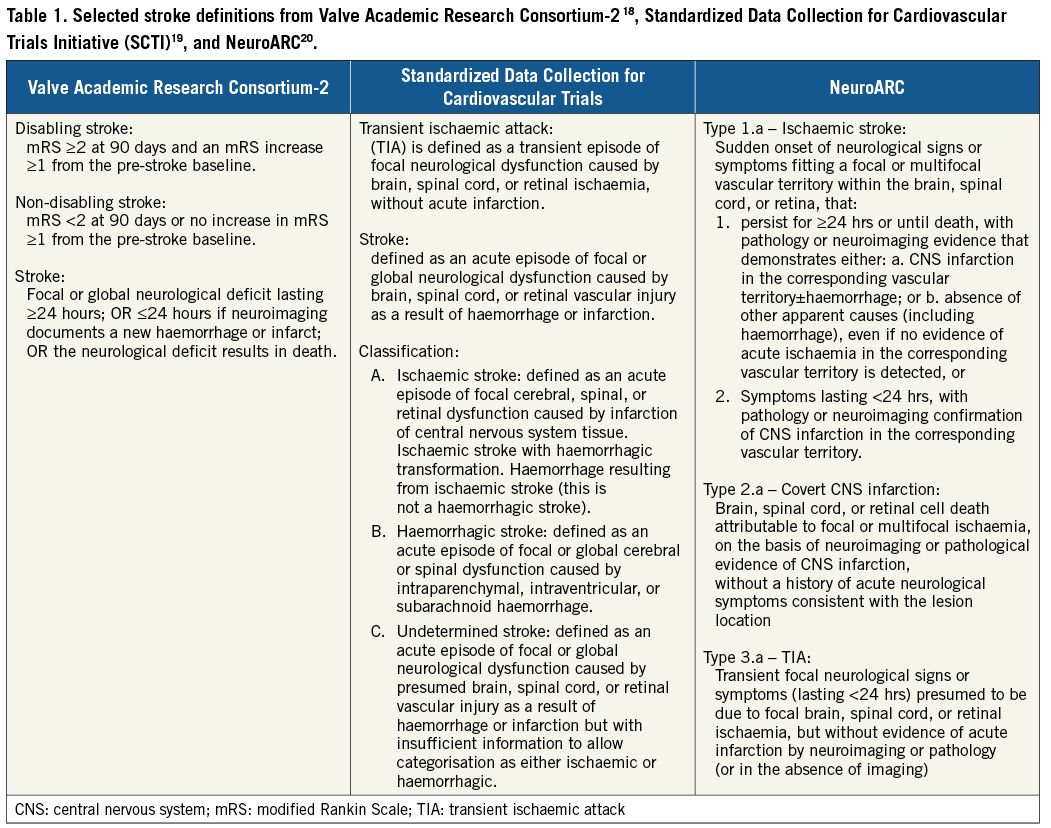
Both patient- and procedure-related factors contribute to the risk of stroke following TAVI. At least half of reported strokes following TAVI are procedure-related and iatrogenic. The most likely causes of procedural embolisation are catheter manipulation within the aorta along with valve and catheter and wire manipulation across the aortic valve (Figure 1)24-27. Characteristics of the TAVI prostheses appear to contribute to cerebral embolisation through various mechanisms including non-steerable TAVI devices being more prone to interact with the aortic arch, whereas balloon-expandable TAVI devices are prone to embolisation during balloon expansion and post-dilation28. The remaining ≈50% of reported strokes are predominantly spontaneous, occurring well beyond the procedure time frame. Primary contributors to spontaneous stroke after TAVI are related to established patient factors, such as age, comorbidities, and atrial fibrillation. Alternative mechanisms for stroke following TAVI include thromboembolism from a variety of mechanisms, which remain poorly understood yet have significant therapeutic implications. For patients at intermediate risk undergoing TAVI in the PARTNER II trial, new-onset atrial fibrillation (NOAF) at 30 days and one year was common (9.1% and 10.1%, respectively), and neurologic events accrued over time (6.4% at 30 days and 10.1% at one year). NOAF has been associated with a twofold to fivefold increased risk of stroke following TAVI29,30. The correlation between NOAF, leaflet motion abnormalities, and periprocedural stroke implicates thrombin as a mediator of ischaemic sequelae. Leaflet motion abnormalities detected by four-dimensional volume-rendered CT resolving after anticoagulation along with the observation of delayed leaflet endothelialisation further hint at a thrombosis-mediated phenomenon31. Both thrombin- and platelet-mediated outcomes are the subject of many ongoing randomised clinical trials. Preventive approaches (Figure 1), including patient selection and therapeutic strategies focused on procedural neuroprotection, and both procedural and long-term adjunctive pharmacology are complementary and target these different underlying aetiologies. A definitive stroke risk model to guide decision making for cerebral embolic protection (CEP) or adjunctive pharmacology use is currently lacking, as cerebral injury is ubiquitous with poorly defined long-term consequences, and major stroke remains unpredictable.
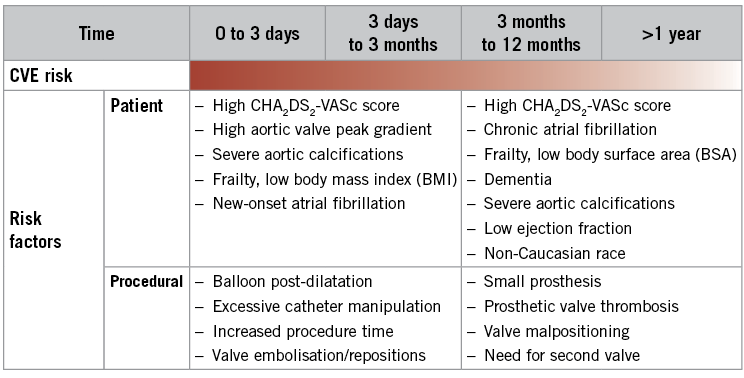
Figure 1. TAVI and stroke: periprocedural and post-procedural risk factors and preventive strategies. Adapted from Dangas et al, 2016 27.
Neuroprotection: preventing procedure-related neurologic injury
Procedure-related cerebral embolisation and neurologic injury are ubiquitous and therefore predictable in all TAVI procedures, resulting in an ≈1 in 10 stroke risk. Initial evidence suggests that procedure-related embolisation is preventable with the use of CEP.
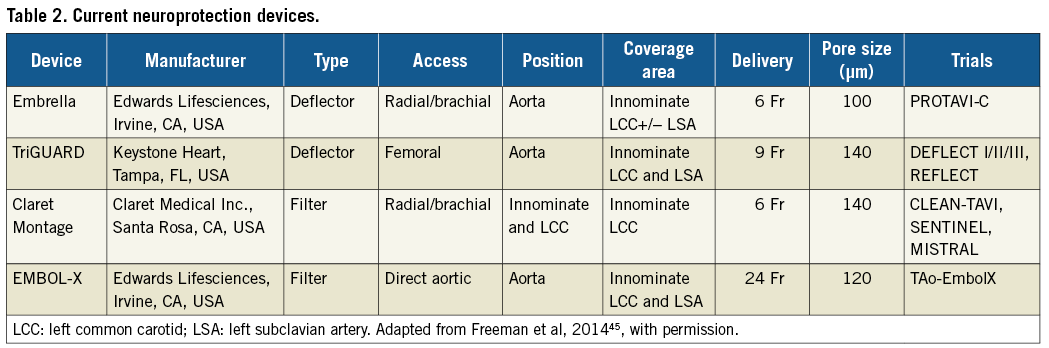
A number of CEP devices exist, varying by access, coverage area, position, sheath size, and pore size (μm) (Table 2). Mechanistically, these devices differ by whether they deflect or capture periprocedural emboli. A systematic review and study-level meta-analysis examined the effect of CEP in TAVI for several outcomes including clinical assessments (NIHSS and MoCA), total lesion volume (TLV) (mm3), number of new ischaemic lesions, and patients with new ischaemic lesions32. Four studies were included in the meta-analysis: CLEAN-TAVI (Claret Embolic Protection and TAVI) trial, DEFLECT-III (A Prospective, Randomized Evaluation of the TriGuard™ HDH Embolic Deflection Device During TAVI) trial, TAo-EmbolX (Intraprocedural Intraaortic Embolic Protection With the EmbolX Device in Patients Undergoing Transaortic Transcatheter Aortic Valve Implantation), and MISTRAL-C (MRI Investigation in TAVI with Claret) trial. CEP was associated with a lower TLV (mm3) (standardised mean difference [SMD] –0.65; 95% CI: –1.06 to –0.25; p=0.002) and fewer new ischaemic lesions (SMD –1.27; 95% CI: –2.25 to –0.09; p=0.03). There was a non-significant trend associated with CEP use towards lower risk for deterioration in NIHSS score at discharge (risk ratio: 0.55; 95% CI: 0.27 to 1.09; p=0.09) and higher MoCA score (SMD 0.40; 95% CI: 0.04-0.76; p=0.03). Although it is a meta-analysis of predominantly first-generation devices with inherent limitations, this study does suggest early evidence of clinical benefit with CEP use during TAVI.
SENTINEL™ (CLARET MEDICAL) (Figure 2A)
MISTRAL-C, a hypothesis-generating study randomising TAVI patients to receive the Sentinel Cerebral Protection System or no CEP, found that CEP use was associated with protection of neurocognition as assessed by the Mini Mental Status Exam (MMSE) and MoCA, and also with a decrease in the number and volume of new MRI lesions33. CLEAN-TAVI was an evaluation of the Claret Montage Dual Filter System, demonstrating that CEP use reduced the volume and size of new brain lesions on MRI two days post TAVI8. The SENTINEL trial is the largest study to evaluate periprocedural CEP use in TAVI patients, randomising 363 patients in a 1:1:1 ratio to an imaging control arm, imaging device arm, and a safety arm. There was no difference in median total new lesion volume assessed by MRI at two to seven days following TAVI. Results from a battery of neurologic and neurocognitive assessments found no significant difference between control and device arms. However, debris was found within the filters of 99% of patients28. A more recent single-centre study of 802 consecutive patients undergoing TAVI with and without CEP demonstrated that CEP use resulted in lower all-cause mortality or VARC-2 defined stroke at seven days (2.1% vs. 6.8%; p=0.01)34. After consideration of all available data, the Claret device (currently CE marked in Europe) recently gained FDA approval in the USA.
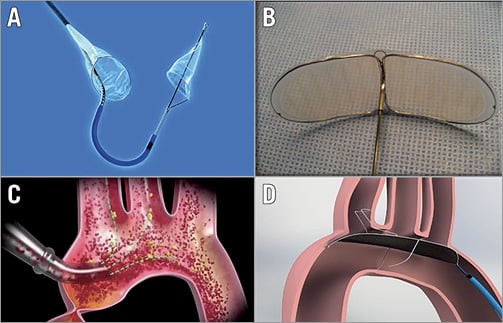
Figure 2. First-generation embolic protection devices. A) Sentinel transcatheter embolic protection device (Claret Medical). B) Embrella (Edwards Lifesciences). C) EMBOL-X (Edwards Lifesciences). D) TriGUARD HDH Device (Keystone Heart). Reproduced from Freeman et al 45, with permission.
EMBRELLA (EDWARDS LIFESCIENCES, IRVINE, CA, USA) (Figure 2B)
The Embrella device was investigated in a pilot study of 52 patients (41 device, 11 control) in which lesion volume at seven days following TAVI was lower in the device arm despite the presence of new ischaemic lesions in all patients in both groups. MoCA scores at 30 days improved in the device arm as well as in the control arm. MMSE scores were unchanged for both groups35. The device is not currently being evaluated in any clinical study.
EMBOL-X (EDWARDS LIFESCIENCES) AND CARDIOGARD (CARDIOGARD LTD., OR YEHUDA, ISRAEL) (Figure 2C)
The EMBOL-X (intra-aortic filtration) and CardioGard (suction-based extraction) devices are positioned within the aorta to capture emboli during open heart surgery. In a trial randomising patients undergoing SAVR to one of two CEP devices versus no CEP, there was no difference in the composite endpoint of freedom from clinical or radiographic CNS infarction at seven days after the procedure. The study was stopped prematurely by the data safety monitoring board due to futility36.
TRIGUARD™ (KEYSTONE HEART, TAMPA, FL, USA) (Figure 2D)
DEFLECT I evaluated the safety and performance of the TriGUARD device and found the device to be safe. DW-MRI demonstrated that new cerebral ischaemic lesion counts were similar to historic controls; however, the per-patient total lesion volume was lower compared to the historic control. The TriGUARD device received CE mark approval on the basis of these results37. DEFLECT III was a multicentre, prospective, randomised study comparing TAVI with the TriGUARD HDH Embolic Deflection Device to TAVI without CEP. In the per-treatment analysis, use of the device was associated with greater freedom from new ischaemic brain lesions (26.9% versus 11.15%), fewer neurologic deficits assessed by NIHSS (3.1% versus 15.4%), and improved MoCA scores at discharge and 30 days6. The first-generation TriGUARD device was evaluated in the US approval REFLECT Phase I trial. The trial was suspended early to reinitiate the REFLECT Phase II trial evaluating the second-generation TriGUARD device (Phase I remains blinded and integral to the REFLECT clinical trial programme).
Neuroprotection devices in development
A number of neuroprotection devices are in early phases of development and early clinical evaluation (Table 3). The TriGUARD 3™ (Keystone Heart) (Figure 3A) is an embolic deflector that covers all three major aortic vessels. It is currently under clinical investigation in the REFLECT trial (NCT02536196), a randomised controlled, US multicentre trial of patients undergoing TAVI with and without CEP. The Emboliner™ Prosheath (Emboline, Inc., Santa Cruz, CA, USA) (Figure 3B) is a dual filter device that captures and removes both cerebral and non-cerebral emboli and is deployed through an existing access site. The Stroke Prevention System SPS (Stroke Prevention Systems, Charleston, SC, USA) is a non-invasive device that fits around the neck. When inflated, it briefly occludes both carotids creating a pressure gradient that deflects cerebral emboli. Point-Guard™ (Transverse Medical, Golden, CO, USA) (Figure 3C) is a dynamic, double-edge sealing deflector and filter, which can conform to variable aortic geometry. Emblok™ (Innovative Cardiovascular Solutions, Kalamazoo, MI, USA) (Figure 3D) is a filter that provides complete coverage of the aortic arch. Early feasibility and safety studies are currently ongoing.
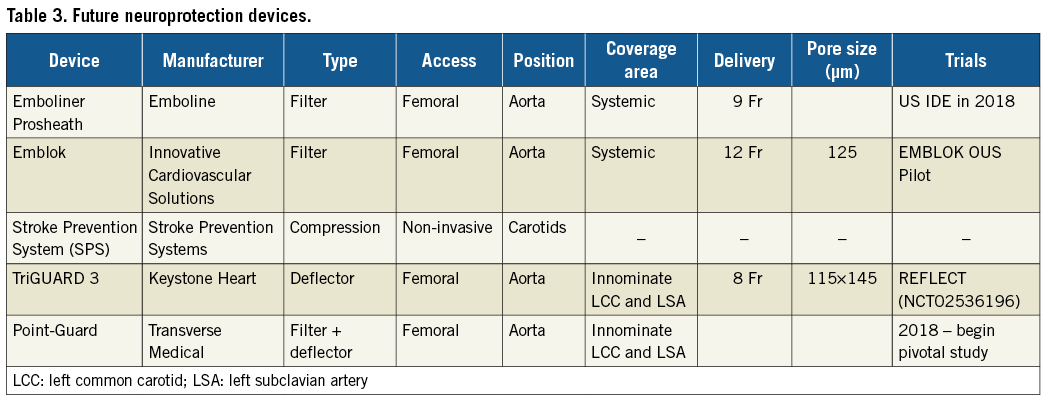
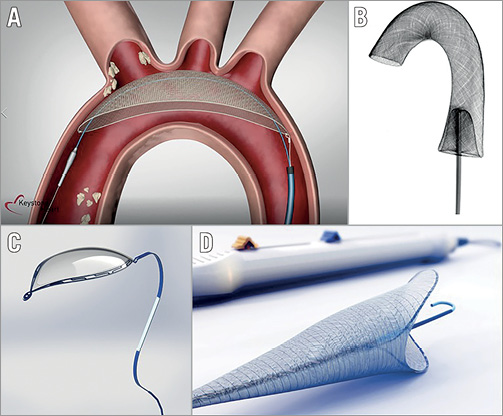
Figure 3. Embolic protection devices under development. A) TriGUARD 3 (Keystone Heart). B) Emboliner Prosheath (Emboline). C) Point-Guard (Transverse Medical). D) Emblok (Innovative Cardiovascular Solutions).
Clinical implications
Initial trial results provide preliminary evidence that CEP is capable of producing measurable neurologic and cognitive benefits. However, randomised trials are needed to determine the magnitude, extent, and duration of these preventive benefits. It is important to note that current CEP devices are safe, do not significantly prolong procedure time, and have demonstrated promise in being effective. In a recent systematic review and meta-analysis, the use of CEP devices demonstrated a trend towards lower risk of death or stroke (RR 0.61; 95% CI: 0.35-1.07; p=0.08)38. The consequences of stroke cannot be written off and thus careful consideration is required when determining whether CEP should or should not be used during TAVI. Finally, there is a lack of data on the effect of CEP on long-term neurocognitive outcomes. Cost-effectiveness evaluation of CEP is currently being established as growing evidence demonstrates early stroke reduction, and its full economic impact will depend on long-term cognition and functional effects. CEP development remains important as an adjunct to TAVI.
Adjunctive pharmacotherapy
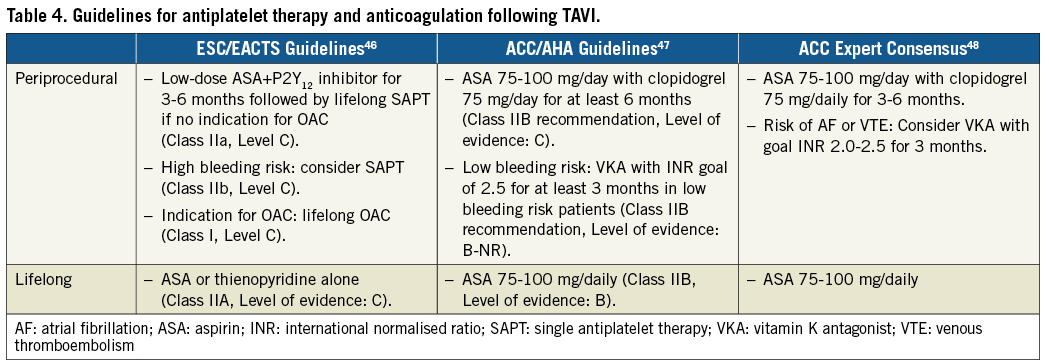
The role of antithrombotic and antiplatelet therapy in prevention of stroke associated with TAVI is complex. Ongoing stroke risk due to valve- and patient-related risk factors such as NOAF or valve leaflet thrombosis must be distinguished from procedure-related risk factors that result in acute procedural embolism of calcified material, which is probably best prevented with CEP. Current guidelines in both the USA and Europe for adjunctive pharmacotherapy are empiric and based on experience rather than an extensive body of evidence (Table 4).
DUAL VERSUS SINGLE ANTIPLATELET THERAPY
The ARTE (Aspirin Versus Aspirin+ClopidogRel Following Transcatheter Aortic Valve Implantation) trial was an open-label, randomised controlled trial comparing aspirin (80 to 100 mg/day) plus clopidogrel (75 mg/day) (dual antiplatelet therapy [DAPT]) to aspirin alone (80 to 100 mg/day) (single antiplatelet therapy [SAPT]) in subjects undergoing TAVI39. Aspirin was started 24 hrs pre-procedure and continued for six months. A loading dose of clopidogrel (300 mg) was started 24 hrs pre-procedure for a transfemoral approach and within 24 hrs post procedure in a non-transfemoral approach. Clopidogrel treatment of 75 mg/day lasted three months. A total of 111 patients were randomised to DAPT and 111 to SAPT. The composite endpoint of death, MI, stroke or TIA, or major or life-threatening bleeding defined by VARC-2 at 90 days occurred in 15.3% of patients in the DAPT arm vs. 7.2% of patients in the SAPT arm (p=0.065). There was no significant difference between DAPT and SAPT for the occurrence of stroke or TIA at three months (2.7% vs. 0.9%, respectively; p=0.31), and all strokes occurred within 30 days following TAVI. There was a significantly increased risk for major or life-threatening bleeding with DAPT compared to SAPT at 30 and 90 days (10.8% vs. 3.6%, respectively, p=0.038). A fixed-effect meta-analysis of 30-day outcomes from three randomised trials comparing DAPT to SAPT in patients undergoing TAVI found no trend for DAPT in reducing stroke and a trend towards increased risk for life-threatening bleeding40.
At face value there seems to be little benefit of DAPT therapy for TAVI; however, the ARTE trial was small (underpowered) and these results do not apply to patients with concomitant AF who may require oral anticoagulation (OAC) or to patients with recent PCI or stent placement. Also, the fact that all stroke events in ARTE occurred within 30 days following TAVI further supports neuroprotection rather than DAPT as a primary preventive method.
PERIPROCEDURAL ANTICOAGULATION
The Effect of Bivalirudin on Aortic Valve Intervention Outcomes (BRAVO)-3 trial was a randomised trial comparing bivalirudin (n=404) to unfractionated heparin (n=398) in patients undergoing transfemoral TAVI41. The co-primary endpoints were not different for bivalirudin versus heparin: major bleeding within 48 hrs or before hospital discharge (6.9% vs. 9.0%, respectively; p=0.27) and net adverse clinical events (all-cause mortality, myocardial infarction, stroke or major bleeding) (14.4% vs. 16.1%, respectively; p=0.50). Additionally, the secondary endpoint of stroke at 30 days was not significantly different for bivalirudin versus heparin (3.5% vs. 2.8%, respectively; p=0.57). A small subset of patients, randomised to bivalirudin (n=29) and unfractionated heparin (n=31), underwent post-procedure DW-MRI imaging42. The proportion of patients with new cerebral emboli on DW-MRI was not different between the two arms, nor was major bleeding (BARC type ≥3). A systematic review and study-level meta-analysis combining data from two non-randomised registries and the BRAVO-3 trial found no significant difference between bivalirudin and heparin for 30-day all-cause mortality (OR 0.97, 95% CI: 0.62-1.52) or stroke (OR 1.23, 95% CI: 0.62-2.46)43. Future and ongoing trials will fill the gap in evidence for optimal periprocedural and post-procedural anticoagulation. Until then, some observational studies provide additional data.
LEAFLET THROMBOSIS, STROKE AND PREVENTION
In a combined analysis of patients undergoing multidetector CT for valvular imaging after TAVI or SAVR from two registries, 106 (12%) of 890 patients had subclinical leaflet thrombosis44. Subclinical leaflet thrombosis was detected more frequently in transcatheter valves compared to surgical valves (13% vs. 4%, respectively). Furthermore, subclinical leaflet thrombosis resolved in 100% of patients (n=36) on a vitamin K antagonist (VKA) and 33% of patients (n=12) on a novel oral anticoagulant (NOAC), while it persisted in 91% of patients (n=20) not receiving anticoagulation (p<0.0001). Ischaemic stroke rates were not different between those with and those without reduced leaflet motion. However, subclinical leaflet thrombosis was associated with increased rates of TIA and the combined endpoint of all strokes or TIAs. Results from this study suggest that OAC may be appropriate in preventing stroke for patients with leaflet thrombosis. These findings require further investigation; several ongoing trials will contribute evidence for the selection of the optimal antithrombotic regimen following TAVI.
ONGOING ANTIPLATELET AND ANTITHROMBOTIC THERAPY TRIALS
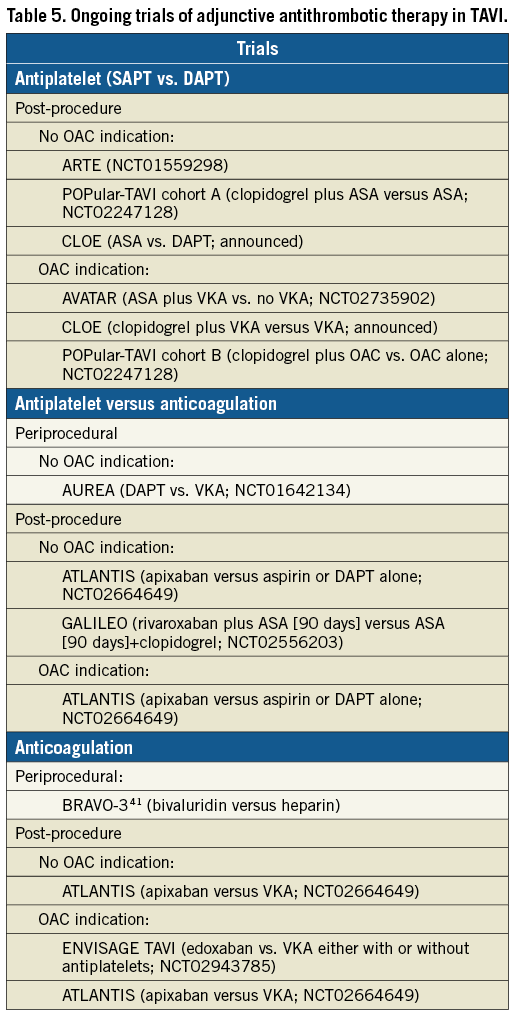
Several studies are investigating various antithrombotic strategies after TAVI. With antithrombotic therapy, the risks of bleeding must be balanced against stroke prevention. Several ongoing trials are investigating optimal antithrombotic regimens following TAVI (Table 5). Primary endpoints for these trials will all be examined >3 months post TAVI and will not provide evidence for periprocedural CVA prevention.
FOR PATIENTS WITH NO INDICATION FOR OAC
ARTE (NCT01559298), POPular-TAVI (NCT02247128), and CLOE (announced) will compare aspirin to DAPT, and three studies are comparing antiplatelet therapy versus anticoagulation therapy: AUREA (DAPT vs. VKA; NCT01642134), GALILEO (rivaroxaban plus aspirin versus DAPT alone; NCT02556203), and ATLANTIS (apixaban versus aspirin or DAPT alone; NCT02664649).
FOR PATIENTS WITH AN INDICATION FOR OAC
Three studies are comparing optimal antithrombotic regimens, AVATAR (aspirin plus VKA vs. no VKA; NCT02735902), POPular-TAVI (clopidogrel plus VKA vs. VKA alone; NCT02247128), and CLOE (clopidogrel plus VKA vs. VKA alone; announced). Two studies are comparing NOAC vs. VKA: ATLANTIS (apixaban versus VKA; NCT02664649) and ENVISAGE-TAVI AF (edoxaban vs. VKA either with or without antiplatelets; NCT02943785).
Conclusions
Stroke after TAVI remains a significant and preventable complication. Risk factors are procedure- and patient-related. Prevention strategies will probably combine neuroprotection and antiplatelet or anticoagulation regimens for selected patient groups. Ongoing trials will fill the evidence gap to inform on the optimal strategy in this growing and increasingly risk-diverse population.
Conflict of interest statement
A. Baumbach has received institutional grant support and has served on the advisory board for Abbott Vascular, MicroPort, Cardinal Health and Sinomed, and has received speaker fees from Keystone Heart and AstraZeneca. A. Lansky has received honoraria, travel expense coverage and institutional research support from Keystone Heart. The other authors have no conflicts of interest to declare.

