Abstract
Aims: Transcatheter aortic valve implantation (TAVI) represents a novel treatment option for inoperable or high surgical risk patients with severe symptomatic aortic valve disease. Recent randomised studies have raised major safety concerns because of increased stroke/transient ischemic attack (TIA) rates with TAVI compared to medical treatment and conventional aortic valve replacement. We aimed to review all currently published literature and estimate the incidence of periprocedural stroke and outcomes in patients undergoing TAVI.
Methods and results: Fifty-three studies including a total of 10,037 patients undergoing transfemoral, transapical or trans-subclavian TAVI for native aortic valve stenosis published between 01/2004 and 11/2011 were identified and included in a meta-analysis. Patients were 81.5±1.8-years-old and had a mean logistic EuroSCORE of 24.77±5.60%. Procedural stroke (<24 h) occurred in 1.5±1.4%. The overall 30-day stroke/TIA was 3.3±1.8%, with the majority being major strokes (2.9±1.8%). During the first year after TAVI, stroke/TIA increased up to 5.2±3.4%. Differences in stroke rates were associated with different approaches and valve prostheses used with lowest stroke rates after transapical TAVI (2.7±1.4%). Average 30-day mortality was more than 3.5-fold higher in patients with compared to those without stroke (25.5±21.9% vs. 6.9±4.2%).
Conclusions: TAVI was associated with average 30-day stroke/TIA rate of 3.3±1.8% (range 0-6%). Most of these strokes were major strokes and were associated with increased mortality within in the first 30 days.
Introduction
Transcatheter aortic valve implantation (TAVI) has been rapidly embraced as a novel treatment option for elderly patients with symptomatic aortic valve disease deemed at high risk for conventional aortic valve replacement (AVR)1,2. The recently published randomised PARTNER (Placement of Aortic Transcatheter Valves) trials have not only demonstrated the superiority of TAVI over standard medical therapy for patients with excessive surgical risk for surgical AVR and considered inoperable3, but also shown comparable outcomes with those at high-risk undergoing AVR4. However, these studies have raised major safety concerns with TAVI, reporting 30-day stroke/transient ischaemic attack (TIA) rates of 6.7% and 5.5%, respectively, in TAVI patients3,4.
The major aim of our meta-analysis was to review all currently published literature and estimate the incidence of periprocedural stroke and outcomes in patients undergoing TAVI.
Methods
DATA SOURCES AND STUDY SELECTION
Using the terms “transcatheter”, “transfemoral”, “transapical”, “percutaneous”, “aortic”, “valve”, and “implantation” a comprehensive search of the English-language medical literature was performed using the MEDLINE database to identify all studies on TAVI. All articles published between January 1, 2004 and November 11, 2011 were initially considered. Bibliographies of the retrieved articles were hand-searched for additional potentially relevant studies. A multistage assessment was used to determine if articles would qualify for the analysis. At the initial stage, only the abstracts were reviewed. Original publications including patients undergoing TAVI for native aortic valve stenosis either by the transfemoral, transaxillary/trans-subclavian, or transapical route were selected for data extraction. Case reports, reports on valve-in-valve TAVI for failed surgical bioprostheses as well as reviews were not included. At the second stage, the full text versions of the selected articles were reviewed for data extraction. Multiple reports of previously listed patients were refined for data on the most recent number of patients or data with most information on clinical characteristics or outcomes to avoid duplicate reporting. Studies were evaluated for the total number of patients provided in the respective publication. If available, data from an individual study were separated into three groups according to the valve device and the access site used: group 1- Medtronic/CoreValve (MCV) ReValving System transarterial (transfemoral and transaxillary/subclavian); group 2- Edwards SAPIEN (ES) transarterial; group 3- Edwards SAPIEN transapical.
DEFINITIONS
Transient ischaemic attack was defined in studies as any reversible neurological deficit with duration of < 24 hours. Procedural stroke/TIA in studies were defined as any neurological event occurring within 24 hours after the procedure. Stroke or TIA were not re-adjudicated for this analysis and any stroke or TIA reported in a given published study was considered to be an event.
STATISTICAL ANALYSIS
Rates of events were calculated as the number of events divided by the number of treated patients with available data. The approach to calculate individual rates for different studies and to combine these rates into a weighted average gives identical results if the weights are defined as the proportion of available patients provided in a specific study5. Results are presented as weighted mean±1 standard deviation.
Results
STUDY SELECTION
A comprehensive literature search identified 748 citations published within the predetermined time span of the search. After careful review, 53 studies3,4,6-56 comprising of a total of 10,037 patients undergoing TAVI met the study criteria and were selected for the current analysis (Table 1).
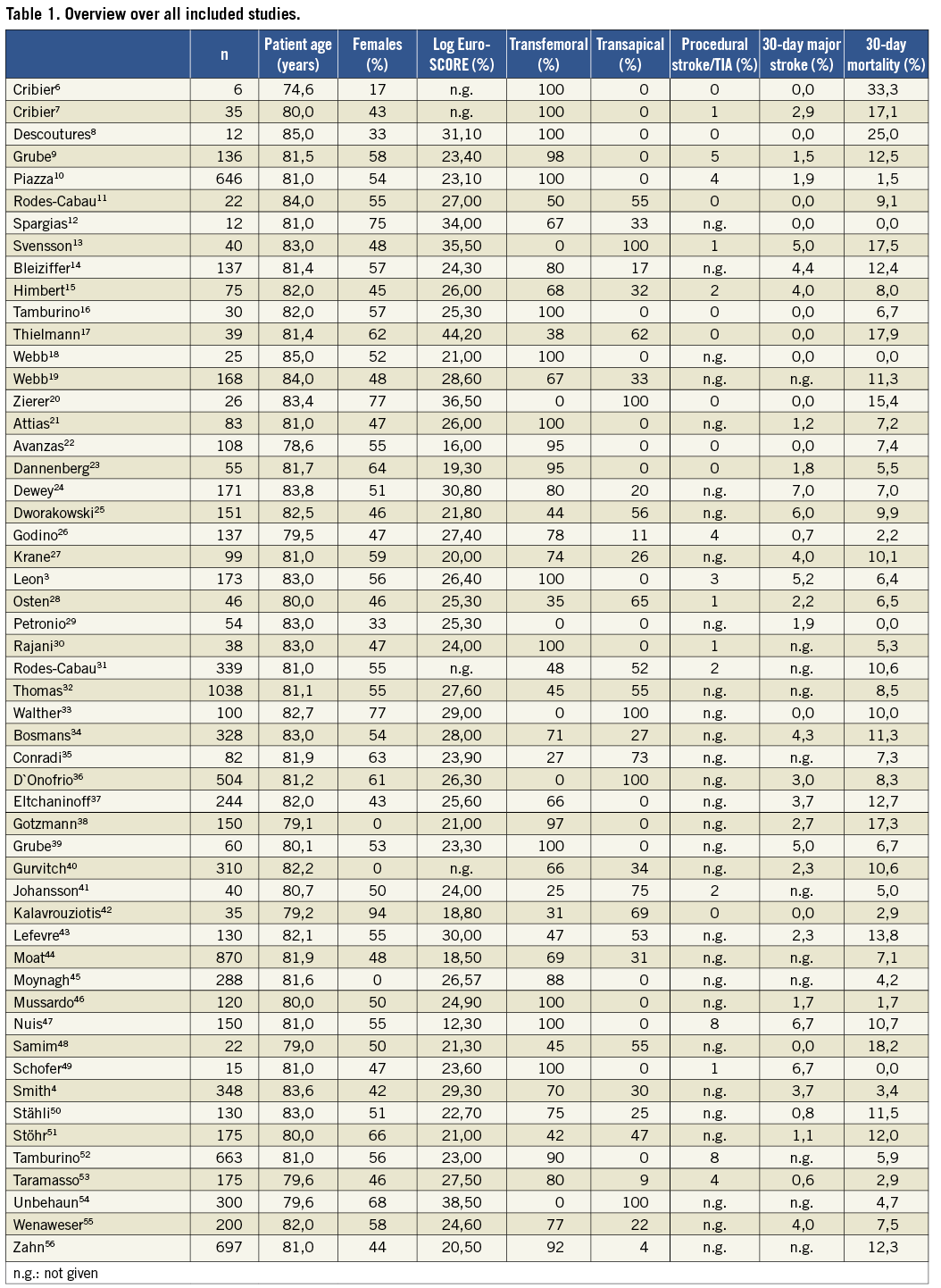
STUDY POPULATION
Characteristics of the patient population are detailed in Table 2. Mean patient age was 81.5±1.8 years with slightly over half being females (53.1±7.6%). The mean logistic EuroSCORE was high at 24.77±5.60%. The Society of Thoracic Surgeons (STS) score was reported in 28/53 (53%) studies and a total of 4293 patients, averaging 12.3±5.0%. In general, neurologic comorbidities were reported rather inconsistently in 32 studies (66%) (Table 2).
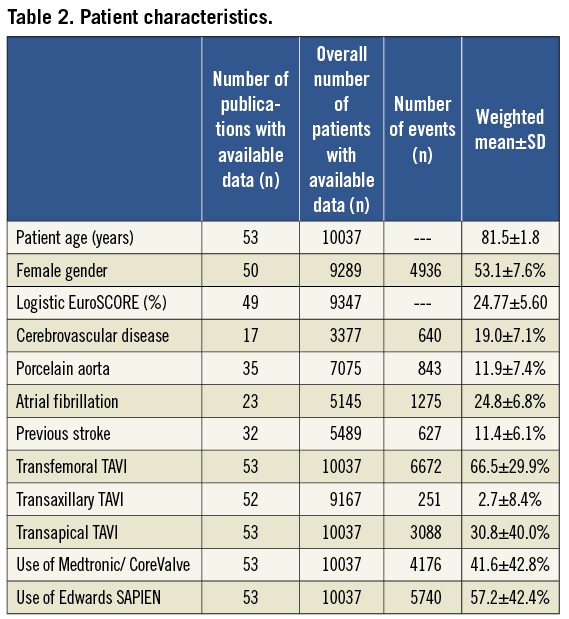
In two-thirds of patients, TAVI was performed via the transfemoral route. In almost one-third of patients, a transapical approach was used. A minority of patients (2.7±8.4%) underwent TAVI using a transaxillary/subclavian access. The ES valve was used in the majority of patients (57.2±42.4%), while the MCV prosthesis was used in the remaining patients. Fifteen of the total of 10,037 (0.15%) patients were enrolled in a first-in-man study using a novel non-metallic aortic valve prosthesis (Direct Flow Medical Inc, Santa Rosa, CA, USA).
INCIDENCE OF STROKE
Forty-two of 53 (79%) studies differentiated between major stroke, minor stroke, and transient ischaemic attack. Procedural stroke (<24 h) occurred in 1.5±1.4% (Table 3). During the first 30 days after TAVI, 334 out of the total of 10,037 patients suffered from stroke or TIA, accounting for an overall 30 day-stroke/TIA rate of 3.3±1.8%. The majority of strokes were major strokes (2.9±1.8%).
The rate of stroke during follow-up was reported by only a few studies and averaged 4.3±1.6% at six months, and 5.2±3.4% at one year, respectively (Table 3).
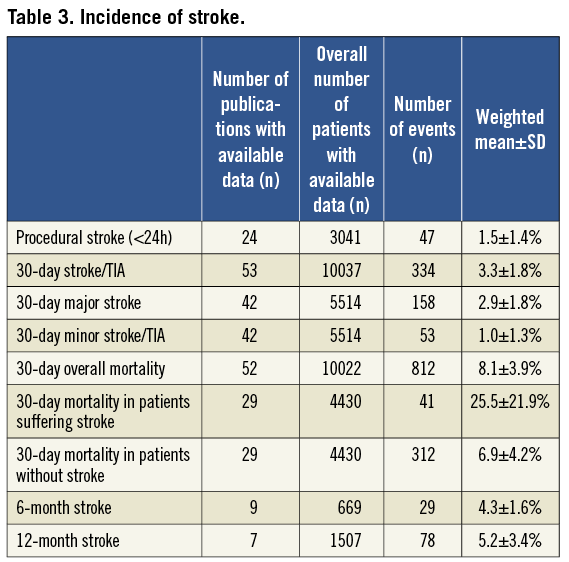
STROKE WITH DIFFERENT TAVI APPROACHES
A total of 3,236 patients underwent transarterial TAVI using the MCV prosthesis (group 1), 1,733 patients transarterial TAVI using the ES valve (group 2), and 2,482 patients transapical TAVI using the ES valve (group 3). Mean age was similar between the groups, but there were significant differences in the logistic EuroSCORE (Table 4). Thirty-day stroke/TIA was lowest in group 3 (2.7±1.4%), higher in group 1 (3.1±2.2%) and highest in group 2 (4.2±2.2%, Figure 1).
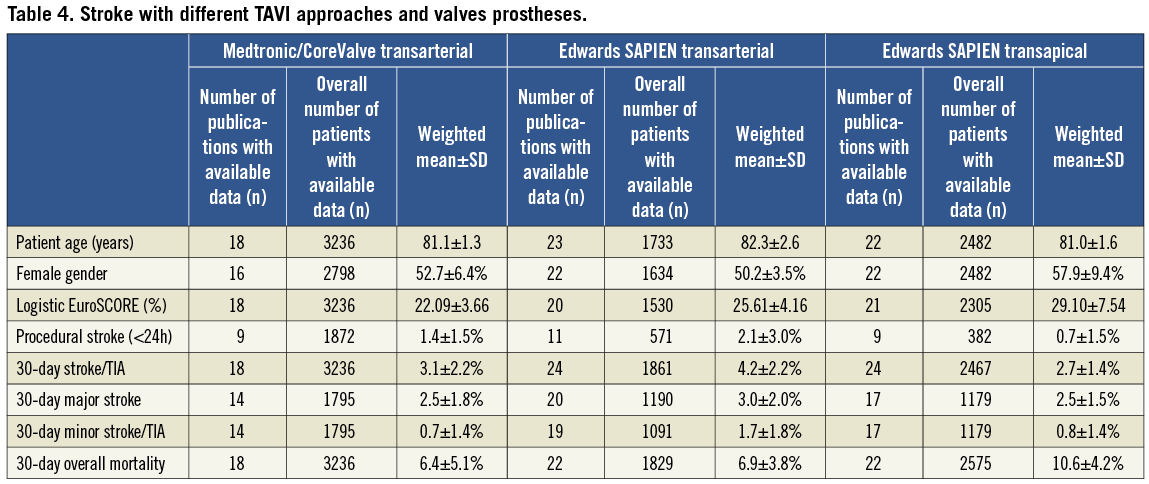
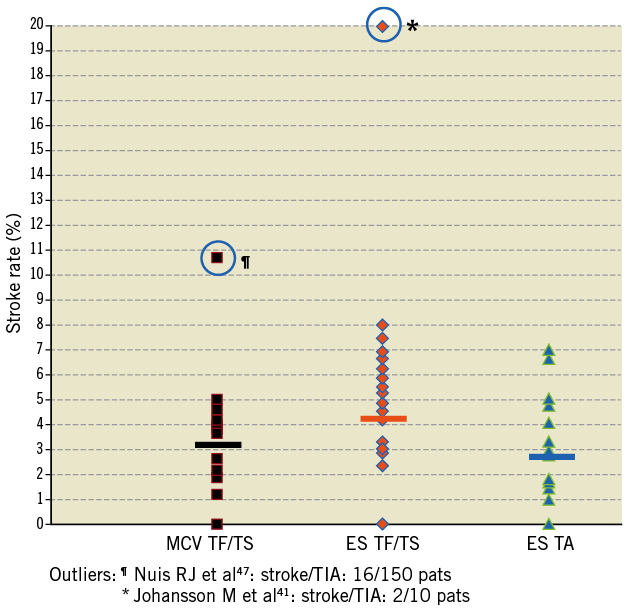
Figure 1. Variation of stroke/TIA rates with different TAVI approaches and prostheses used.
STROKE AND MORTALITY
Overall, 30-day mortality was 8.1±3.9%. Mortality in patients suffering from stroke was more than 3.5-fold higher than in non-stroke patients (25.5±21.9% vs. 6.9±4.2%, Table 3). Persistent neurologic deficits were reported in nine studies comprising a total of 536 patients. In these nine studies, 24 of 536 (4.5%) patients suffered from stroke/TIA at 30 days. Thirteen of these 24 strokes were major strokes with persistent neurological deficits reported in 12 (50%) patients.
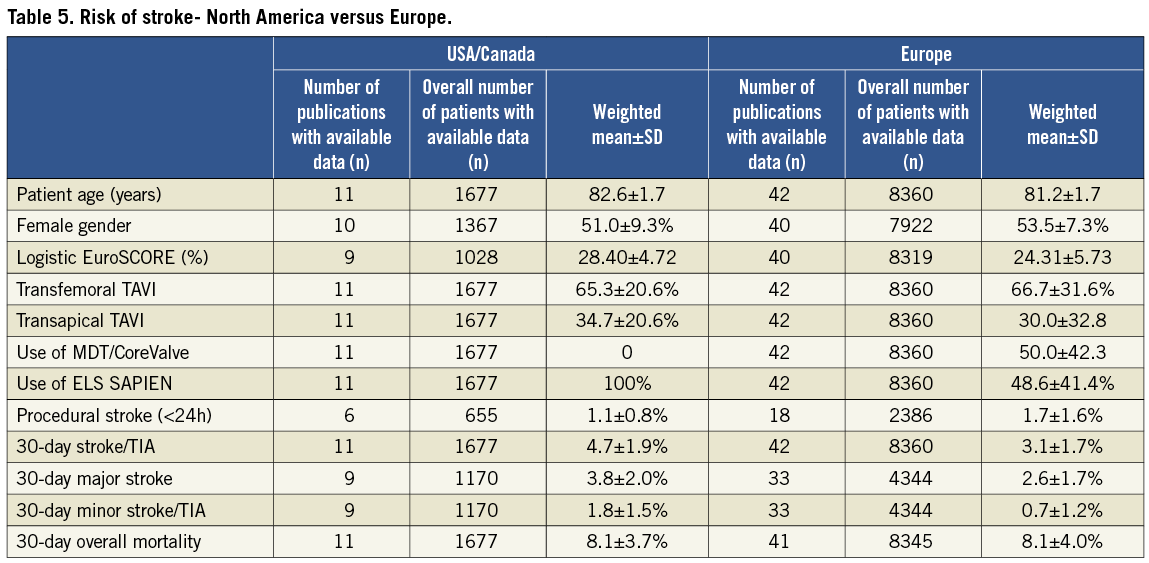
DIFFERENCES IN TAVI STROKE RATES BETWEEN EUROPE AND NORTH AMERICA
Publications on TAVI from European institutions included 8,360 patients (mean age: 81.2±1.7 years), while the remaining 1,677 patients were reported from the US/Canadian groups (mean age: 81.2±1.7 years, Table 5). In European publications, 50% of patients received MCV valves while in US/Canadian publications only the ES valve was used. At 30 days, average stroke/TIA rate in US/Canadian publications was 4.7±1.9% and in European publications it was 3.1±1.7%. Overall mortality was similar at 30 days (8.1±3.7% vs. 8.1±4.0%).
Discussion
Stroke is the most dreaded complication of open surgical and interventional catheter procedures shown to be associated not only with increased acute mortality but also increased morbidity, physical disability and resource use. It is therefore not surprising that elderly patients who are currently referred for TAVI are often as concerned about stroke as about dying during the procedure. The recent reports of increased stroke risk reported with TAVI in the two randomised PARTNER trials have also heightened the awareness of the risk of this event among physicians performing TAVI and stimulated them to understand the factors associated with and mechanisms underlying this risk as well as for evaluating strategies for reducing it. However, currently few data exist in literature regarding this.
The present meta-analysis of published TAVI literature comprising a total of 10,037 patients revealed an average 30-day rate stroke/TIA of 3.3±1.8%. This risk was not confined only to the periprocedural period, but follow-up of these patients indicated that TAVI patients continued to experience this event during the first year after TAVI, with the current analysis demonstrating an average rate of 5.2±3.4%. Even more importantly, our data suggested that six of every seven stroke events were deemed as major event rather than minor attesting to its high association with major disability in those who survived this event. In the very few studies that provided information on persistent neurological sequelae at 30-days, these were reported in 50% of patients with stroke. In addition, significant heterogeneity in stroke/TIA rates were noted depending on the type of valve and approach used to implant them: The lowest stroke/TIA rates were observed in patients undergoing transapical implantation of Edwards SAPIEN valves and those undergoing transfemoral implantation of Medtronic/CoreValve prostheses. In contrast, the transfemoral implantation of the unsheathed, large-bore Edwards SAPIEN valve portended the highest risk of stroke/TIA. Finally, our analysis corroborated findings of previous studies, showing that 30-day mortality among patients who suffered peri-interventional stroke was approximately 3.5-fold higher than in those without stroke (25.5±21.9% vs. 6.9±4.2%).
It is important to view the current data on the risk of stroke after TAVI in the context of the underlying inherent stroke risk in the population similar to that referred for this procedure, but who are treated with standard medical therapy (inoperable patients) or who undergo conventional high-risk surgical AVR. The recent PARTNER B study showed that inoperable patients with calcific aortic valve stenosis receiving standard medical management have a stroke/TIA risk without TAVI or surgery of approximately 1.7% at 30 days and 4.5% at one year3. In this trial, the risk of stroke/TIA was higher among those undergoing TAVI occurring in 6.7% and 10.6% of patients at 30 days and one year3. For conventional AVR, the overall rate of stroke for isolated AVR is approximately 1.5% based on the Society of Thoracic Surgeons (STS) database, but may be increased to 2-4% in older or high-risk patients57. Elbardissi et al58 recently reported a stroke rate of 4% among 259 octogenarian TAVI candidates (age: 84±3 years, EuroSCORE 11%) undergoing minimally invasive surgical AVR. In the randomised cohort A of the PARTNER trial4, the risk of stroke/TIA in the patients undergoing conventional AVR was 2.4% at 30 days and 4.3% at one year, whereas this was 5.5% and 8.3% in those undergoing TAVI. These data may suggest that the manipulation of the calcified and diseased aortic valve and atherosclerotic aorta increases the likelihood of dislodgement of debris resulting in higher acute risk of stroke in those undergoing TAVI compared with those with similar risk profile who are managed medically. Similarly, while manipulation of aortic valve and aorta is inherent during both TAVI and surgical AVR, it is likely that perhaps the surgical AVR allows better removal of debris and potential for less embolisation because of cross-clamping of the aorta. In contrast to the increased risk of acute neurological events with TAVI, longer-term risk is not increased by it and remained similar to those managed medically or surgically and determined by underlying risk profile of patients with aortic valve disease.
MECHANISMS OF UNDERLYING STROKE
Catheter manipulations of the calcified and diseased aortic valve may cause embolisation of aortic debris or thrombotic material resulting in stroke or TIA. Omran et al59 showed early that retrograde passage of the aortic valve even with small-bore diagnostic catheters for haemodynamic evaluation carried a risk of clinically silent cerebral embolism as detected by magnetic resonance imaging (MRI) as well as apparent neurologic deficits. New hyper-intense cerebral lesions were found in 22 (22%) out of 101 patients undergoing diagnostic catheterisation, with three (3%) patients developing clinically apparent stroke/TIA59. Transcatheter aortic valve implantation requires additional procedural steps such as valvuloplasty, retrograde passage of the aortic arch and the stenosed valve with semi-rigid, large-bore delivery catheters containing the crimped stent valve, and finally crushing of the native leaflets to the aortic wall by the metallic stent frame60. Therefore, the risk for periprocedural cerebral embolism is expected to be intuitively higher with this procedure. Recent mechanistic studies using diffusion-weighted cerebral MRI have demonstrated new cerebral lesions in 68%-91% of patients after TAVI60-64. These lesions were usually multiple and dispersed in both hemispheres in a pattern suggesting cerebral embolisation. Interestingly, the volumes of the new cerebral lesions were significantly smaller in TAVI patients compared with patients undergoing conventional AVR, of whom one patient developed a clinically apparent stroke postoperatively60.
Despite the high frequency of new cerebral lesions on MRI in these series, only few patients had acute clinically apparent stroke/TIA after TAVI. Additionally, the long term clinical significance of these cerebral lesions remains unknown. Kahlert et al60 have shown that new cerebral foci on MRI were not associated with apparent neurological events or even measurable deterioration of neurocognitive function during 3-month follow-up. Even more interesting was the finding that on three-month MRI follow-up there was no residual signal change associated with the majority (80%) of the foci detected during the periprocedural period60.
Our analysis provided further support for the association of the intensity of catheter manipulations with the incidence of stroke/TIA during TAVI, showing differences in the incidence of this event with different approaches for TAVI. Previous investigators33 have speculated that antegrade, transapical TAVI may be advantageous over retrograde, transarterial TAVI with respect to neurological events. Transapical TAVI requires only minimal catheter manipulation of the ascending aorta and arch, which may be a substantial source of embolism in elderly patients with advanced atherosclerosis33. Indeed, our analysis showed that transapical TAVI using the Edwards SAPIEN valve was associated with the lowest stroke/TIA rate (2.7±1.4±% at 30 days) while retrograde transarterial implantation of the same Edwards SAPIEN valve portended a higher risk (4.2±2.2%). Interestingly, retrograde transarterial implantation of the 18 Fr Medtronic/CoreValve prosthesis which protects the crimped valve during delivery by an overlying sheath was associated with a reduced stroke /TIA rate (3.1±2.2% stroke/TIA at 30 days) compared to the relatively bulkier 22-24 Fr Edwards SAPIEN valve implanted via the same route. It remains to be established if transfemoral delivery of the newer and less bulky Edwards SAPIEN XT valve (18-19 Fr delivery system) is associated with a lower incidence of stroke compared to that observed using current valve.
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Clearly with any new procedure, refinements in technique and technology are expected based on initial experience with it. This is particularly true when a disease condition (i.e., aortic valve disease) has poor prognosis when managed medically and the procedure to treat it is particularly easier and less invasive (i.e., TAVI) compared with conventional approach (i.e., surgical AVR). Thus, the finding of increased risk of acute stroke with TAVI compared with similar risk patients that are medically managed or have surgical AVR should stimulate future research to identify the underlying risk factors and mechanism of stroke so that appropriate strategies may be designed and evaluated to improve this risk with the newer procedure. Large carefully designed registries are needed to evaluate the risk factors for stroke with TAVI. While current evidence points to cerebral embolism of aortic valve or atherosclerotic debris from the aorta at the time of catheter manipulation as the major mechanisms underlying the underlying stroke risk with TAVI, the contributions of other mechanisms to stroke risk such as hypoperfusion of brain during balloon valvuloplasty or implantation of the valve (in elderly patients with already low cerebral reserve) needs to be evaluated in future as implications for treatments may be different. Refinements in technology with smaller delivery systems for valves may have the potential for reducing the incidence of stroke with this procedure. The impact of different antithrombotic regimen (including bivalirudin compared with heparin, role of glycoprotein GpIIbIIIa receptor antagonists or pre-treatment with potent antiplatelet agents such as clopidogrel, prasugrel or ticagrelor) as well as the efficacy/effectiveness of cerebral embolic protection devices in reducing the risk of stroke in TAVI patients also remains to be determined. It is clear from current data that long-term stroke risk (i.e., beyond 30 days) was similar among patients with aortic valve disease irrespective of the initial management strategy employed and may have the highest potential of being impacted by better control of hypertension and management of dyslipidaemia. Most of these proposed theories for reduction of stroke remain speculative at this time necessitating careful evaluation in future. Finally, the higher stroke risk observed with TAVI compared with medical treatment of high-risk patients with symptomatic aortic stenosis should not be perceived by physicians as prohibitive for referring appropriate patients for this procedure, given the unequivocal improvement in functional status and mortality with this procedure compared with those managed medically3.
STRENGTHS AND LIMITATIONS
Our meta-analysis analysed stroke/TIA rates from 53 published studies comprising 10,037 patients undergoing TAVI that did not specifically focus on stroke during this procedure. Thus, our analysis may be subject to publication bias as many addressing these issues may have not been published. The overall rate seen in most observational studies was lower than that seen in the PARTNER trials accounting for the overall lower rate of stroke in this meta-analysis and this may be related to ascertainment bias as well as under-reporting in many studies. Given that MRI lesions were much more common in TAVI patients and that 87% of all strokes within 30 days were major stroke, the likelihood of under-reporting of minor stroke, particularly in observational studies cannot be entirely excluded. Strokes were not defined uniformly in all studies. The uniform use of a standardised definition of neurologic events after TAVI according to the Valve Academic Research Consortium (VARC) recommendations may help overcome this issue in future65. Most studies lack control groups of medically managed patients or patients undergoing surgical AVR and comparisons were made with historical controls. The denominator differed for each variable in the current analysis, as not all variables included in the present analysis were reported uniformly by all studies. Therefore, it was not possible to evaluate potential predictors of periprocedural stroke/TIA. Finally, inference regarding superiority of particular TAVI device over others or different approaches used for delivery of the devices or differences in outcomes by countries should be made with caution.
Conclusions
TAVI was associated with an average 30-day stroke/TIA rate of 3.3±1.8% (range 0-6%). Most of these strokes were major strokes and were associated with a 3.5-fold increased mortality with one of four patients with stroke dying in the first 30 days. These data should stimulate studies of both devices and strategies of adjunctive pharmacotherapy to reduce the frequency and severity of strokes after TAVI in the future.
Conflict of interest
H. Eggebrecht is a clinical proctor for Medtronic/CoreValve and Edwards Lifesciences and has received honoraria payment. P. Kahlert has received honoraria payment as a clinical proctor from Edwards Lifesciences. The other authors have no conflict of interest to declare in relation to this article.

