
Abstract
Aims: Transcatheter aortic valve replacement (TAVR) has become the procedure of choice for inoperable patients and a safe alternative to surgical aortic valve replacement (SAVR) among moderate-risk patients. We used meta-analysis to compare the incidence of cerebrovascular events amongst patients undergoing TAVR and SAVR in randomised controlled trials (RCT).
Methods and results: Our search revealed five RCT published between 2011 and 2017 with a total of 5,414 patients. Data were summarised as Mantel-Haenszel relative risk (RR) and 95% confidence intervals (CI). The risk of major stroke (RR 0.89, 95% CI: 0.53-1.51), all strokes (RR 0.85, 95% CI: 0.59-1.22) and all cerebrovascular events (RR 0.94, 95% CI: 0.75-1.17) was comparable between patients undergoing TAVR and SAVR at 30 days of follow-up. The risk of all strokes (RR 0.92, 95% CI: 0.69-1.22), major stroke (RR 0.92, 95% CI: 0.62-1.37) and all cerebrovascular events (RR 1.03, 95% CI: 0.79-1.33) was comparable between TAVR and SAVR at one year of follow-up. The incidence of major stroke (RR 1.02, 95% CI: 0.64-1.61), all strokes (RR 1.12, 95% CI: 0.78-1.62) and all cerebrovascular events (RR 1.23, 95% CI: 0.91-1.66) was comparable between TAVR and SAVR between 30 days and one year of follow-up.
Conclusions: In our meta-analysis of RCT comparing TAVR and SAVR, we showed comparable risk of major stroke, all stroke and all cerebrovascular events.
Abbreviations
CI: confidence interval
NOTION: Nordic Aortic Valve Intervention Trial
PARTNER: Placement of Aortic Transcatheter Valves Trial
PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses
RR: relative risk
SAVR: surgical aortic valve replacement
SURTAVI: Surgical Replacement and Transcatheter Aortic Valve Implantation trial
TAVR: transcatheter aortic valve replacement
TIA: transient ischaemic attack
Introduction
Transcatheter aortic valve replacement (TAVR) has become the treatment of choice for inoperable patients with symptomatic, severe aortic stenosis and is a viable alternative to surgical aortic valve replacement (SAVR) for high and intermediate surgical risk patients. However, there are several adverse effects associated with TAVR, prime among them being stroke and transient ischaemic attack (TIA)1-7. At 30 days, around 4% of patients undergoing TAVR experience strokes and 5.7% of patients experience a stroke and/or TIA8. A sub-analysis of the CoreValve trial (and Continued Access Study) demonstrated that the incidence of stroke may be over 8% at one year9.
While acute cerebrovascular events (stroke and TIA) are undoubtedly significant adverse events after TAVR, there are several areas of uncertainty related to their occurrence. The comparative incidence of stroke in patients undergoing TAVR and SAVR has often been questioned. The landmark PARTNER trial (2011) reported a higher incidence of neurological events in patients undergoing TAVR as compared to SAVR5. In stark comparison, the SURTAVI trial (2017) showed that patients undergoing SAVR had a greater incidence of stroke at 30 days (5.6% vs. 3.4%) and one year (6.9% vs. 5.4%)10. Also, it has been shown that the risk for post-TAVR stroke may be biphasic, with early and late strokes having different pathophysiology. Existing meta-analyses have focused on traditional temporal endpoints of 30 days and one year and the comparative risk in between these time points needs to be evaluated11-13. Additionally, recent literature suggests that patients undergoing TAVR may have an increased risk of subclinical leaflet thrombosis as compared to SAVR patients, which may be associated with stroke and TIA, although this has not been established14,15. We aimed to compare the incidence of cerebrovascular events at 30 days, one year and between 30 days and one year amongst patients undergoing TAVR and SAVR in randomised controlled trials (RCT) using meta-analysis. We also systematically reviewed the reporting of cerebrovascular endpoints and risk factors for cerebrovascular events among the individual RCT.
Methods
SEARCH STRATEGY AND INCLUSION CRITERIA
We included all randomised trials comparing neurological outcomes in TAVR and SAVR with a minimum one year of follow-up. Our search strategy and inclusion criteria are detailed in Supplementary Appendix 1 and Figure 1.
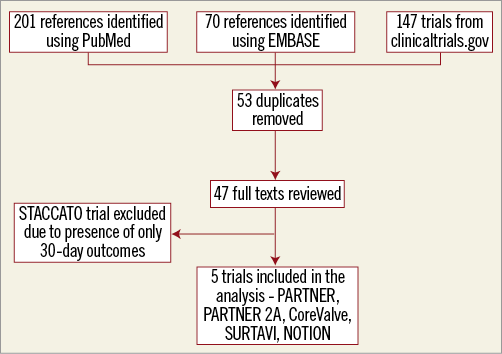
Figure 1. Description of the search strategy for the meta-analysis.
STUDY ENDPOINTS
The primary outcome of interest for our study was all strokes at one-year follow-up. Secondary outcomes included: (1) major stroke at 30 days, (2) all stroke (a composite of major and minor strokes) at 30 days, (3) all cerebrovascular events (a composite of all major strokes, minor strokes and TIA) at 30 days, (4) all stroke (a composite of major and minor strokes) at one year, (5) all cerebrovascular events (a composite of all major strokes, minor strokes and TIA) at one year, (6) major stroke between 30 days and one year, (7) all strokes (a composite of major and minor strokes) between 30 days and one year, and (8) all cerebrovascular events (a composite of all major strokes, minor strokes and TIA) between 30 days and one year. The terms major stroke/disabling stroke and minor stroke/non-disabling stroke are used interchangeably in this paper.
SENSITIVITY ANALYSIS
We further explored the heterogeneity in each primary and secondary analysis by means of sensitivity analysis using the “one study removal” method, wherein the effect of removal of individual studies on the overall results was assessed.
DATA ABSTRACTION AND INDIVIDUAL STUDY QUALITY APPRAISAL
Details of the data abstraction strategy, review of study protocols and risk of bias of included studies using the standardised criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions is provided in Supplementary Appendix 2 and Supplementary Table 1 16.
STATISTICAL ANALYSIS
Categorical dichotomous data were summarised across treatment arms using the Mantel-Haenszel risk ratio (RR) along with 95% confidence intervals (CI). We evaluated heterogeneity of effects using Higgins’ I-squared (I2) statistic. A fixed effects model was used except in cases where heterogeneity was significant (defined as I2 >25%). If the heterogeneity was significant, a random effects model was used. A cut-off of 25% was used as values >25% signify moderate to severe heterogeneity and indicate that variability across studies cannot be attributed to chance17. To address publication bias, we used Egger’s test18,19. Funnel plot analysis was not carried out as the number of included studies was small (<10). Comprehensive Meta-Analysis v. 3.3.070 (Biostat, Englewood, NJ, USA) was used for the meta-analysis. A two-tailed p-value of 0.05 was considered significant for all our analyses.
Results
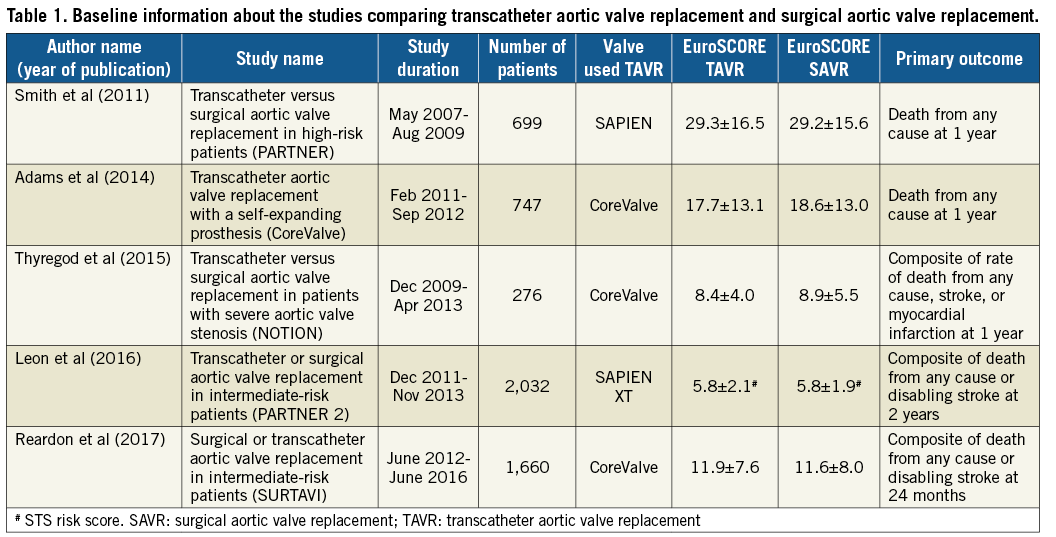
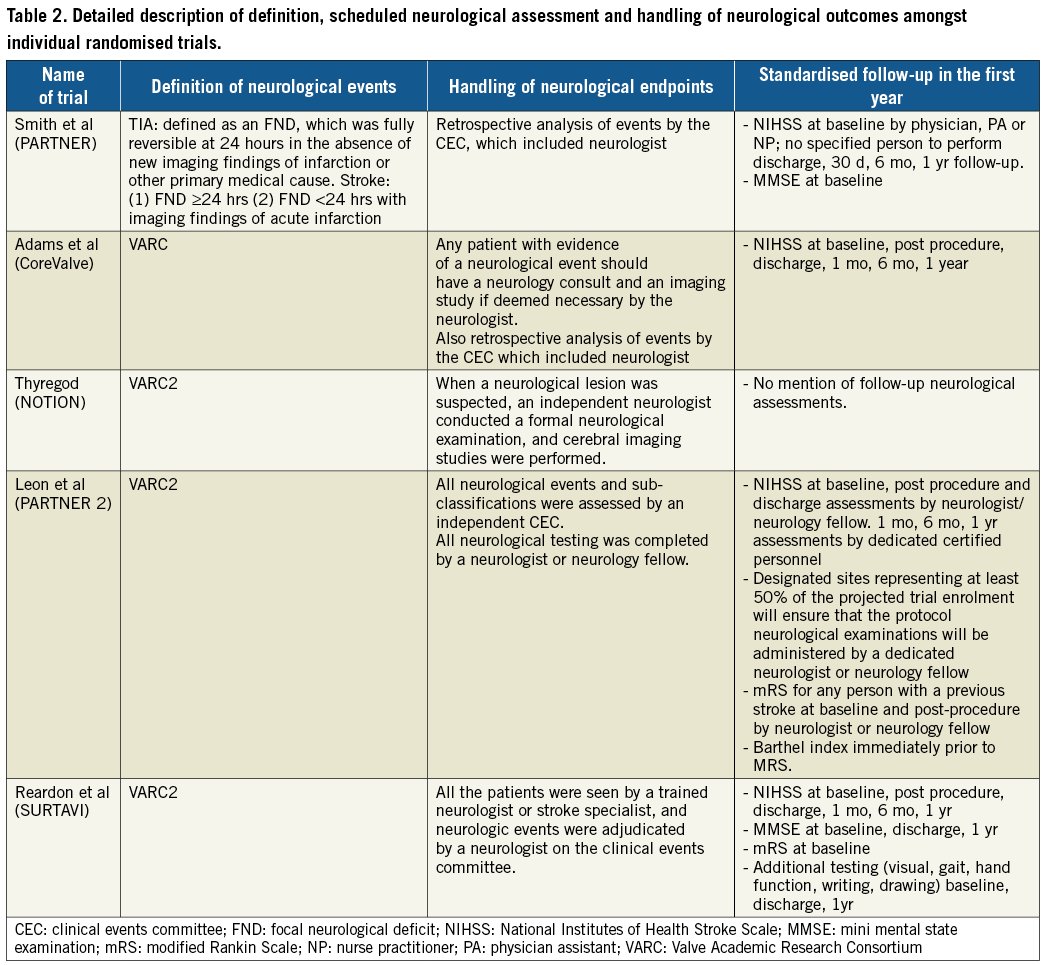
Our search results yielded five RCT published between 2011 and 2017 with a total of 5,414 patients (TAVR 2,755, SAVR 2,659)4,5,10,20,21. Details of the individual RCT are included in Table 1. A detailed description of stroke risk factors among individual studies is available in Supplementary Table 2. Details of definition, scheduled neurological checks and handling of neurological events in individual studies are provided in Table 2. Throughout this study, the first PARTNER trial5 is referred to simply as the PARTNER trial, whereas the second PARTNER trial4 is referred to as the PARTNER 2A trial.
1. Primary outcome (all strokes at one year of follow-up).There was no significant difference between patients undergoing TAVR and SAVR (RR 0.92, 95% CI: 0.69-1.22, I2=42%) (Figure 2).
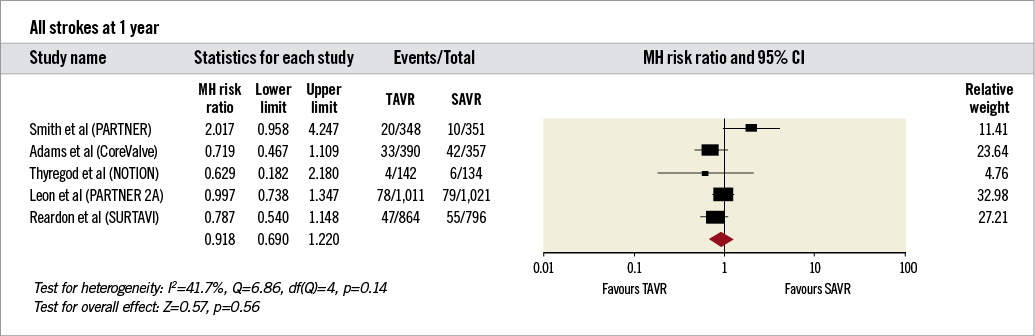
Figure 2. Forest plot comparing risk of all strokes in patients undergoing transcatheter and surgical aortic valve replacement. The diamond indicates the overall summary estimate for the analysis. The centre of the diamond represents the point estimate and the width represents the 95% confidence interval.
2. Secondary outcomes at 30 days (Figure 3A-Figure 3C).The incidence of major stroke (RR 0.89, 95% CI: 0.53-1.51, I2=55%), all strokes (RR 0.85, 95% CI: 0.59-1.22, I2=44%) and all cerebrovascular events (RR 0.94, 95% CI: 0.75-1.17), I2=47%) was comparable between patients undergoing TAVR and SAVR.
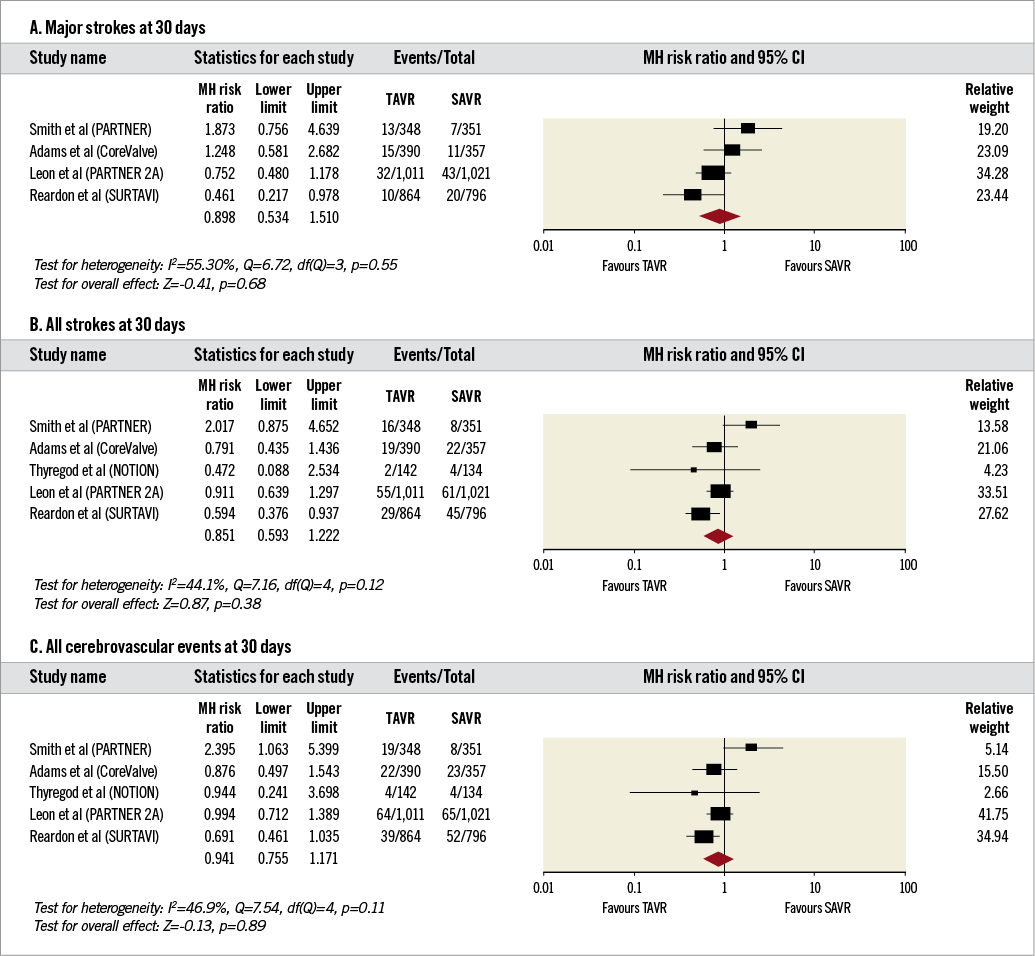
Figure 3. Forest plots comparing risk of events in patients undergoing transcatheter and surgical aortic valve replacement at 30 days. A) Risk of major stroke. B) Risk of all stroke. C) Risk of all cerebrovascular events. The diamond indicates the overall summary estimate for the analysis. The centre of the diamond represents the point estimate and the width represents the 95% confidence interval.
3. Secondary outcomes at one year (Figure 4A, Figure 4B).The incidence of major stroke (RR 0.92, 95% CI: 0.62-1.37, I2=51%) and all cerebrovascular events (RR 1.03, 95% CI: 0.79-1.33, I2=46%) was comparable between TAVR and SAVR.
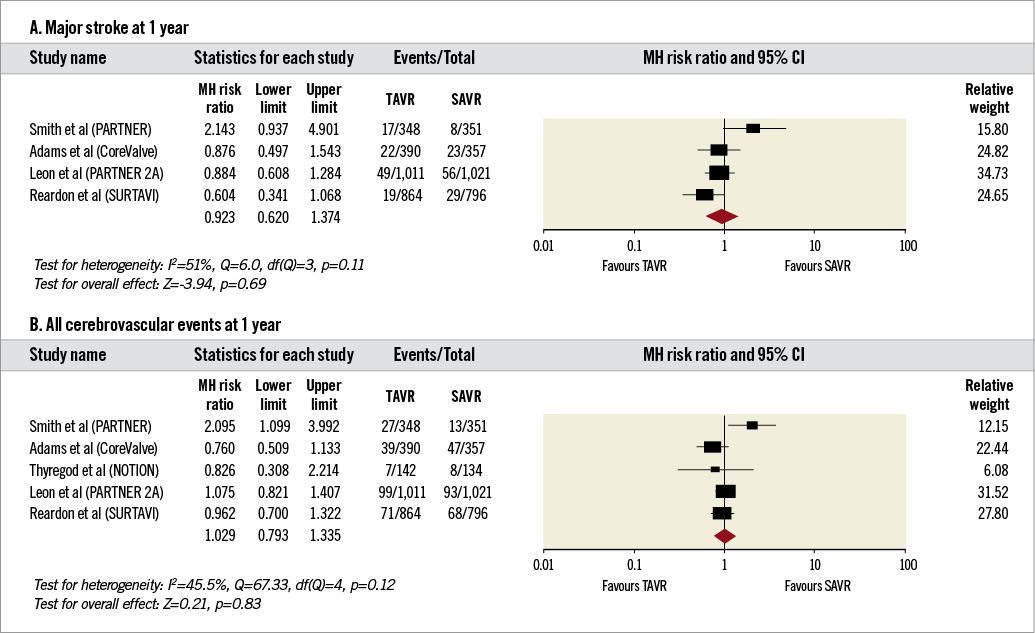
Figure 4. Forest plots comparing risk of major stroke and all cerebrovascular events in patients undergoing transcatheter and surgical aortic valve replacement at one year. A) Risk of major stroke. B) Risk of all cerebrovascular events. The diamond indicates the overall summary estimate for the analysis. The centre of the diamond represents the point estimate and the width represents the 95% confidence interval.
4. Secondary outcomes between 30 days and one year (Figure 5A-Figure 5C).
The incidence of major stroke (RR 1.02, 95% CI: 0.64-1.61, I2=24%), all strokes (RR 1.12, 95% CI: 0.78-1.62, I2=9%) and all cerebrovascular events (RR 1.23, 95% CI: 0.91-1.66, I2=21%) was comparable between TAVR and SAVR.
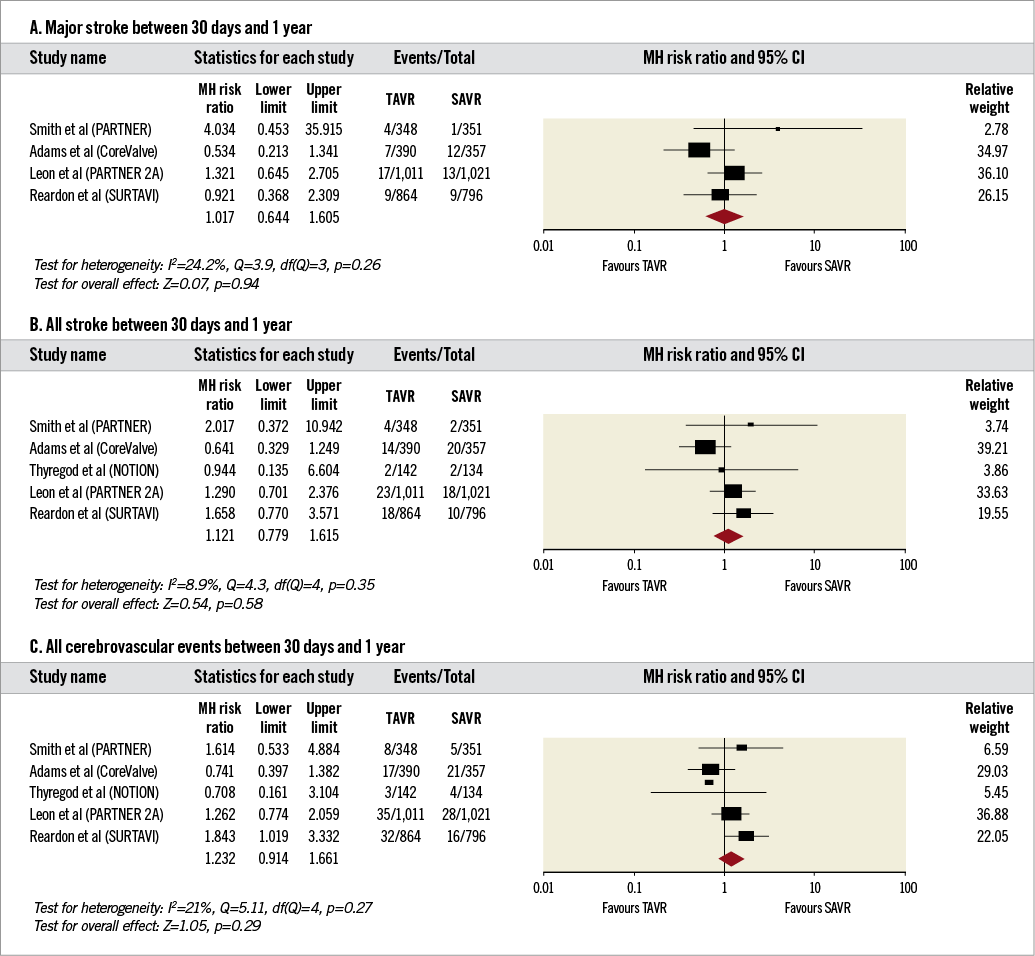
Figure 5. Forest plots comparing risk of events in patients undergoing transcatheter and surgical aortic valve replacement between 30 days and one year of follow-up. A) Risk of major stroke. B) Risk of all stroke. C) Risk of all cerebrovascular events. The diamond indicates the overall summary estimate for the analysis. The centre of the diamond represents the point estimate and the width represents the 95% confidence interval.
SENSITIVITY ANALYSIS (Supplementary Figure 1)
Sensitivity analysis using the “one study removal” method did not result in any significant changes in effect on any of the outcomes except (1) all strokes at 30 days and (2) all cerebrovascular events at one year. For the outcome of all strokes at 30 days of follow-up, we observed that, after exclusion of the PARTNER trial, the risk was significantly reduced in patients undergoing TAVR (RR 0.76, 95% CI: 0.59-0.98). Exclusion of the other studies individually for this outcome did not alter the overall effect. For the outcome of all cerebrovascular events between 30 days and one year, exclusion of the CoreValve trial showed that patients undergoing TAVR had an increased risk of all cerebrovascular events (RR 1.42, 95% CI: 1.01-2.02) between 30 days and one year. Exclusion of both CoreValve and PARTNER trials resulted in a borderline increased risk of all cerebrovascular events (RR 1.41, 95% CI: 0.98-2.03). Exclusion of the other studies individually for this outcome did not alter the overall effect.
PUBLICATION BIAS
Egger’s test did not reveal evidence of publication bias for any of the other primary, secondary or supplementary analyses.
Discussion
In this meta-analysis of five RCT with over 5,400 patients randomised to TAVR and SAVR we showed that major strokes, all strokes, and all cerebrovascular events were comparable between the two groups at 30 days, one year and between 30 days and one year. Additionally, our sensitivity analyses revealed that removal of the PARTNER trial resulted in a lower risk of all strokes in patients undergoing TAVR, whereas removal of the CoreValve study resulted in an increased risk of all cerebrovascular events between 30 days and one year in those undergoing TAVR. To the best of our knowledge, this is the most up-to-date comparative analysis of cerebrovascular events in TAVR and SAVR. Also, it is the most comprehensive review of trial protocols with regard to cerebrovascular event definition, detection, and monitoring (Table 2).
A wide variability exists in reporting of stroke risk across studies. Often, the reporting of these adverse events is dependent on identification by treating clinicians in retrospective studies or based on self-reporting of events in prospective registries. In fact, in a sub-analysis of the CoreValve trial, Kleiman et al noted that the 8.4% one-year incidence of stroke in their report was markedly higher than the 4.1% in the TVT registry (one year) and 3.9% in the FRANCE registry (six months)9,22,23. For this reason, we chose to analyse only RCT wherein a clear structure, comparative baseline risk and thorough protocol-based reporting of clinical events would allow us to investigate the true incidence of stroke and systematically to investigate differences in trial protocols that could result in differential reporting of cerebrovascular events.
In a comprehensive analysis of neurological events in the PARTNER trial, it was shown that the probability of stroke at 30 days was 3.8% in the transfemoral cohort and 2.7% in the transapical cohort. Thereafter, it increased slowly to 5.4% and 6.9% for the transfermoral cohort, and 4.1% and 7% for the transapical cohort at 1 year and 3 years, respectively9,24. Furthermore, it has been suggested that procedural and anatomical factors may be responsible for this early stroke risk24. The risk for stroke in patients randomised to the TAVR arm was substantially higher in the PARTNER trial, whereas in SURTAVI the incidence of cerebrovascular events was lower in the TAVR group at 30 days, and one year of follow-up5,10. In the PARTNER trial, although the increased risk of major stroke at one year in patients undergoing TAVR did not reach statistical significance, the risk of all cerebrovascular events was significantly increased in the TAVR arm (8.3% vs. 4.3%, p=0.04). This was probably driven by the increased rates of TIA in the TAVR cohort. Of note, our sensitivity analysis revealed that risks of cerebrovascular events are in fact lower in the TAVR group after removal of the PARTNER trial. There are several possible reasons for these findings. PARTNER was the only trial amongst those included in our analysis that did not require neurological evaluation in patients with stroke/TIA to be completed by a trained neurologist and/or a neurology fellow. Additionally, baseline National Institutes of Health Stroke Scale (NIHSS) assessment was completed in this study by a physician (not necessarily a neurologist) or by a nurse practitioner/physician assistant. In comparison, this assessment was carried out by a neurologist/neurology fellow in the PARTNER 2A trial (Table 2). These differences in trial methodology could perhaps have introduced an ascertainment bias. It is also important to recognise that the PARTNER trial did not require routine post-procedural NIHSS evaluation (first follow-up in the absence of stroke was at the time of discharge). This, if present, may have allowed enhanced identification of subtle neurological findings and therefore reduced the risk for ascertainment bias for early stroke. These findings should also be kept in mind while designing future clinical trials, and post-procedural evaluation for stroke should form part of standard protocol for studying cerebrovascular events in trials evaluating cardiovascular procedures.
We also found that, with removal of the CoreValve study, there was an increased risk of all cerebrovascular events in patients undergoing TAVR (p=0.045) between 30 days and one year of follow-up. With additional removal of the PARTNER trial, the increased risk remained of borderline significance (p=0.07). Since the risk of major stroke and all strokes does not change with sensitivity analysis, we can postulate that this increased risk is due to a higher incidence of TIA in patients undergoing TAVR. It is possible that this increased risk of TIA is secondary to subclinical leaflet thrombosis occurring between 30 days and one year. In a recently published report from the SAVORY and RESOLVE registries, Chakravarty and colleagues reported a significantly higher incidence of subclinical leaflet thrombosis in patients undergoing TAVR, which was subsequently associated with a higher risk for TIA15. Additionally, it is possible that the microembolic nature of cerebrovascular events after TAVR may result in early recovery of neurological function and therefore an increased risk of TIAs rather than major or minor strokes.
Limitations
While our meta-analysis provides insight into the neurological events following TAVR and SAVR, there are several limitations to our analysis. First, this is a meta-analysis performed on study-level data and therefore individual patient risk of cerebrovascular events could not be addressed. Second, given the limited number of studies, we were unable to explore the effect of baseline patient and procedural characteristics (such as valve and access type) on outcomes using meta-regression techniques. It is for the same reason that we were unable to carry out subgroup analysis by TAVR access and valve type. Third, the earliest time point of 30 days in our study may be marginally beyond the time frame for early phase of stroke risk (<10 days) as suggested by current literature9. Additionally, both changes in valve technology over time and operator experience may have impacted on the results but could not be accounted for in this analysis. However, we feel that our selective analysis of only randomised patients (with comparable baseline characteristics), large pooled sample size of patients, standardised definitions of neurological outcomes amongst individual studies, and extensive subgroup and sensitivity analysis provide robustness to our study.
Conclusions
In our meta-analysis of RCT comparing TAVR and SAVR, we showed that the risk of major stroke, all stroke and all cerebrovascular events is comparable between the two groups at 30 days and one year.
| Impact on daily practice Our meta-analysis will allow interventional cardiologists to inform their patients undergoing TAVR adequately of their risk of cerebrovascular events. This will be of special value in risk versus benefit discussions in patients who may be potential surgical candidates. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Search strategy and inclusion criteria.
Supplementary Appendix 2. Data abstraction and individual study quality appraisal.
Supplementary Table 1. Risk of bias assessment among various studies.
Supplementary Table 2. Risk factors for cerebrovascular events in individual studies.
Supplementary Figure 1. Sensitivity analysis.
To read the full content of this article, please download the PDF.

