Abstract
Aims: In a prospective randomised trial we aimed to compare transapical transcatheter aortic valve implantation (a-TAVI) with surgical aortic valve replacement (SAVR) in operable elderly patients.
Methods and results: The study was designed as a randomised controlled trial of a-TAVI (Edwards SAPIEN heart valve system; Edwards Lifesciences, Irvine, CA, USA) vs. SAVR. Operable patients with isolated aortic valve stenosis and an age ≥75 years were included. The primary endpoint was the composite of all-cause mortality, cerebral stroke and/or renal failure requiring haemodialysis at 30 days. After advice from the Data Safety Monitoring Board, the study was prematurely terminated after the inclusion of 70 patients because of an excess of events in the a-TAVI group. The primary endpoint was met in five a-TAVI patients (two deaths, two strokes, and one case of renal failure requiring dialysis) vs. one stroke in the SAVR group (p=0.07). In the a-TAVI group, one patient was converted to SAVR because of an abnormally positioned heart, and four patients were re-operated with open heart surgery because of annulus rupture (n=1), severe paravalvular leakage (n=2), and blockage of the left coronary artery (n=1). In the SAVR group, one patient was converted to TAVI because of a large intra-thoracic goitre.
Conclusions: Given the limitations of a small prematurely terminated study, our results suggest that a-TAVI in its present form may be associated with complications and device success rates in low-risk patients similar or even inferior to those found in high-risk patients with aortic valve stenosis. This will probably change in the near future with improved catheter based devices and better pre-procedural assessment.
Introduction
Surgical aortic valve replacement is the golden standard for treating aortic valve stenosis in operable patients.1 According to The PARTNER Trial, transcatheter aortic valve implantation (TAVI) reduces mortality in patients with severe aortic stenosis who are not candidates for surgical aortic valve replacement (SAVR).2 Furthermore, in high-risk patients, TAVI and SAVR seem to imply a similar 1-year risk of all-cause mortality.3 Based on this pivotal randomised clinical trial and TAVI registries4-12 with promising short- and longer-term results, the number of TAVI procedures has increased dramatically over the last five years. TAVI procedures may be performed by a femoral approach or by a small thoracotomy through the apex of the heart (a-TAVI). Other access options are the axillary13 and the direct aortic14 approach. The a-TAVI procedure has primarily been used in patients with stenosed iliac and femoral arteries, prohibitive of insertion of large-lumen catheters. The a-TAVI is a safe and predictable procedure, but has been associated with more adverse events than femoral procedures, probably because of patient selection bias in favour of the femoral approach.
The purpose of the present randomised clinical trial was to compare a-TAVI with SAVR using biological valve prostheses in operable elderly patients with severe aortic valve stenosis.
Methods
Patients
The study was planned as an academic prospective multicentre clinical trial in the Nordic region with a 1:1 randomisation of a total of 200 patients to a-TAVI vs. SAVR. The first patient was included November 2008. After inclusion of 11 patients, we experienced three potentially severe adverse events in the a-TAVI group (one case of left main occlusion, one case of aortic rupture and one case of up-stream valve embolisation). The study was put on hold, and the Data Safety Monitoring Board (DSBM) contacted. It was decided to increase the age limit for inclusion to ≥75 years and to exclude patients with prior heart surgery. This was done to increase the general risk of the study cohort and to enable a swift conversion to SAVR, if needed. After inclusion of 70 patients, the study was terminated prematurely after advice from the DSBM. This decision was based on a general impression of too many adverse events and procedure-related complications following the a-TAVI treatment. The last patient was included May 2011. Two centres participated: the Departments of Cardiothoracic Surgery and Cardiology, Aarhus University Hospital (59 patients) and the Departments of Thoracic Surgery and Cardiology, Odense University Hospital (11 patients). At study initiation, both centres had performed >40 TAVI procedures.15 For a patient flow chart see Figure 1. The presence of coronary artery disease and high surgical risk were the main reasons for exclusion from study participation. All patients were evaluated at weekly Heart Team valve meetings with participation of cardiologists, cardiac surgeons and anaesthesiologists. The patients received detailed information on the study and the different treatment modalities by a study coordinator and a cardiac surgeon before randomisation.
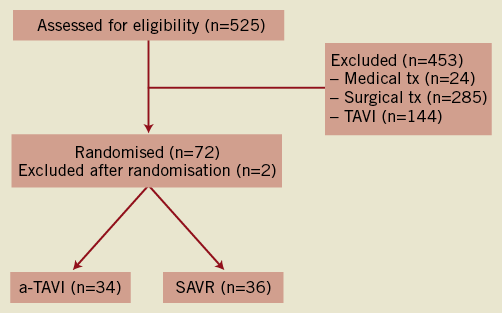
Figure 1. Flow diagram of patients with aortic valve stenosis aged ≥75 years at the enrolling centres. SAVR denotes surgical aortic valve replacement and a-TAVI, transapical transcatheter aortic valve implantation.
Per protocol, there was an echocardiographic assessment at one month and one year. At study termination, we decided to perform a 3-month contact as an out-patient visit.
The study complied with the Declaration of Helsinki and was approved by the ethics committee of the Region of Midtjylland. All patients provided written, informed consent before participation in the trial.
Criteria of inclusion and exclusion
Criteria of inclusion: significant valvular aortic stenosis (valve area <1 cm2), age initially ≥70, later ≥75 yrs; condition accessible both by SAVR and a-TAVI; expected survival >1 year following successful treatment; and patient acceptance of participation in the study as well as in the scheduled follow-up investigations. We used age as our major criterion of inclusion, because age is a simple and well defined parameter closely related to surgical risk.
Criteria of exclusion: coronary artery disease to be treated by percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG); previous myocardial infarction, and previous PCI within 12 months. Previous heart surgery became a criterion of exclusion during the study. The need for other heart surgery (i.e., mitral or tricuspid valve surgery), emergency surgery (within 24 hours of indication for surgery), unstable cardiac condition (requiring an assist device, inotropes or i.v. nitrates in operating room), ongoing infection requiring antibiotics, stroke within one month, reduced pulmonary function (FEV1 <1l or <40% of expected), renal failure to be treated by haemodialysis, allergy to acetylsalicylic acid, clopidogrel, prasugrel or x-ray contrast material.
Endpoints and definitions
The primary endpoint was the composite of 30-day all-cause mortality, major stroke, and renal failure requiring dialysis.
Secondary endpoints included: all-cause death, cardiac death, stroke, myocardial infarction, New York Heart Association (NYHA) function class, SF-36 composite physical and mental functional scores, echocardiographic parameters (aortic valve area, peak aortic valve gradient, aortic valve leakage, left ventricular ejection fraction), duration of hospital stay, operation for bleeding, and permanent pacemaker treatment.
For endpoint definitions, we used the Valve Academic Research Consortium recommendations.16 All endpoints were adjudicated by an independent endpoint committee.
Procedure and study device
The Heart Team selected patients for the study based on history, physical examination, transthoracic echocardiography, coronary angiography and lung function test. Following oral and written information, patients who accepted participation were examined with transoesophageal echocardiography (TOE), and aortography, to ensure technical feasibility both for SAVR and a-TAVI.
The a-TAVI procedures were performed at a cardiac catheterisation laboratory by two cardiac surgeons and an interventional cardiologist. A heart lung machine was present, in case of urgent need for haemodynamic support or conversion to SAVR. The patients were fully sedated and extensively haemodynamically monitored. A 23 or 26 mm Edwards SAPIEN™ Transcatheter Heart Valve (THV) prosthesis (Edwards Lifesciences) was introduced catheter-based via the apex of the heart, through a left mini thoracotomy. The incision was guided by echocardiographic visualisation of the left ventricular apex. The THV was advanced antegradely over the balloon pre-dilated native aortic valve. After ensuring correct position by TOE and fluoroscopy, the THV was implanted during rapid pacing by expansion of a balloon catheter within the valve. Ventricular pacing was performed via temporary myocardial electrodes (160-200 beats per minute). A catheter for postoperative local analgesia was placed intra-pleurally and a chest tube for drainage inserted before closure of the thoracotomy.
Surgical aortic valve replacement was performed through a sternotomy during cardiopulmonary bypass. The native valve was resected and the aortic annulus measured to ensure the correct bioprosthesis size. Felt-armed sutures were placed sub-annularly and the valve prosthesis tied into place. The performance of the bioprosthesis was checked perioperatively using TOE. The PERIMOUNT™ aortic heart valve (Edwards Lifesciences) was recommended by protocol.
Study design
Randomisation and data collection
The 1:1 randomisation between a-TAVI and SAVR was implemented using the web-based clinical trials support system, “TrialPartner” (PCI Research, Aarhus University Hospital, Skejby, Denmark). TrialPartner permits, with a personal log-in, 24-hour randomisation. Data was entered in the electronic case report form of TrialPartner, a secure server based system with security that exceeds the demands and guidelines by the National Data Protection Agency.
Sample size
Based on the Western Denmark Heart Registry SAVR data on patients aged >70 years from 1998 through 2008, we anticipated a primary endpoint rate of 13.5% in the SAVR group. The estimated event rate of 2.5% in the a-TAVI group was based on our experience from non-operable patients with significantly higher risk than the study population. At study initiation, our a-TAVI event rates were 0%15. Given an alpha of 5% and a beta of 80%, 96 patients should be included in each group to document the difference. Therefore, we planned for inclusion of 200 patients in the study.
Statistical methods
Outcomes were analysed by intention-to-treat. Distributions of continuous variables in the a-TAVI and SAVR groups were compared using either the two-sample t-test or the Mann-Whitney U-test, depending on whether the data followed a normal distribution. We compared distributions of categorical variables using the Chi-square test. The tests were two-sided, and a p-value of 0.05 considered significant. Differences in discrete variables are given both as numbers and percentages.
Funding
The study was an academic study, designed and carried out by the involved cardiac surgeons, cardiologists and anaesthesiologists at Aarhus University Hospital, and Odense University Hospital, and primarily funded by the participating hospitals. Further, there was a study grant from the Danish Heart Association. There was no industry involvement.
Results
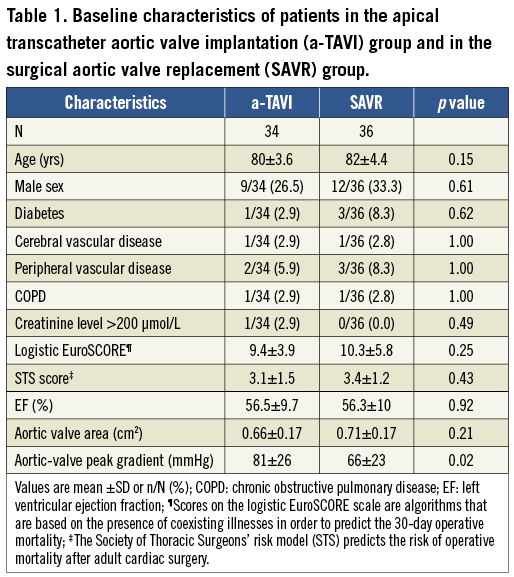
A total of 72 patients were randomised. Two patients were excluded after randomisation; one patient declined participation, and the other unexpectedly met the exclusion criteria of impaired pulmonary function. Thus, the study population consisted of 34 patients in the a-TAVI and 36 patients in the SAVR group. Patients randomised to SAVR had lower peak aortic valve gradient preoperatively. Otherwise, the study groups were well matched in baseline characteristics (Table 1).
The primary endpoint of 30-day all-cause mortality, stroke or renal failure was met in five (14.7%) patients in the a-TAVI group; one death on the waiting list, one death following treatment for left coronary artery obstruction, two major thrombotic/embolic strokes, and one case of renal failure. In the SAVR group, one (2.8%) patient fulfilled the primary endpoint criterion (a major perioperative thrombotic/embolic stroke). The difference in primary endpoint rates was statistically insignificant (p=0.07), Table 2.
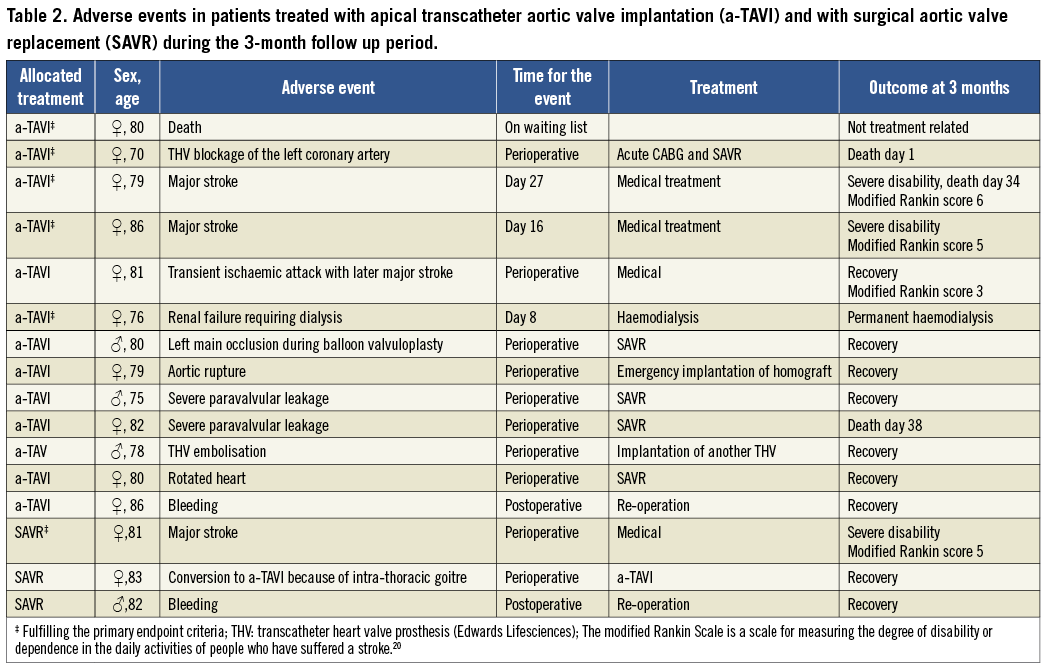
The VARC defined16 device success was 79% in the a-TAVI group vs. 100% in the SAVR group (p=0.004). The adverse events in the two treatment groups at three months are shown in Table 2. Two more a-TAVI patients died; one of the above-described stroke patients died after one month and one patient after SAVR for severe paravalvular leakage. In the a-TAVI group, one patient experienced a transient ischaemic attack, which later developed into a major stroke. We detected no procedure-related or spontaneous myocardial infarctions in the study groups as defined by the Valve Academic Research Consortium.16 Three patients received a permanent cardiac pacemaker: two a-TAVI patients and one SAVR patient. Mean hospital stay was 8.8±6.7 and 7.6±2.4 days (ns) in the a-TAVI and SAVR groups, respectively.
Echocardiographic assessment before and after the index procedure was performed in 28 a-TAVI and 36 SAVR patients. The mean aortic valve area increased (a-TAVI from 0.65±0.16 to 1.39±0.28 cm2, SAVR from 0.71±0.17 to1.29±0.27 cm2) and peak aortic gradient decreased (a-TAVI from 81±26 to 20±6 mmHg, SAVR 66±23 to 24±11 mmHg) significantly in both groups, but slightly more in the a-TAVI than in SAVR treated patients. There was significantly more minimal and moderate/severe paravalvular leakage in the a-TAVI group than in the SAVR group (Figure 2). In two a-TAVI patients, the leakage indicated a re-operation with SAVR.
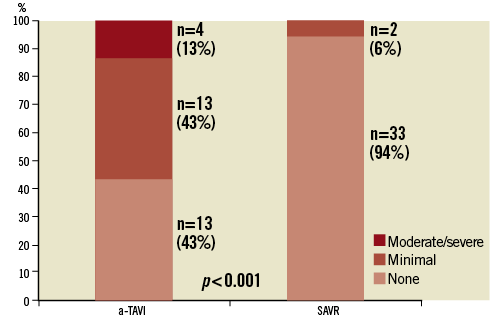
Figure 2. Paravalvular leakage after transapical transcatheter aortic valve implantation (a-TAVI) vs. surgical aortic valve replacement (SAVR).
NYHA functional class (Figure 3) and composite physical and mental scores (Table 3) before treatment and at three months follow-up were similar in the two groups. Within both treatment groups, NYHA functional class increased significantly after treatment.
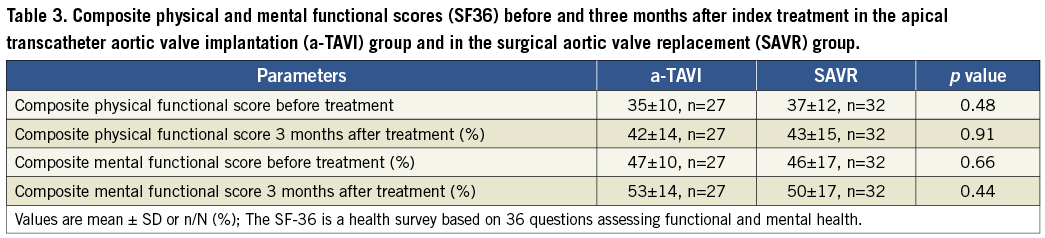
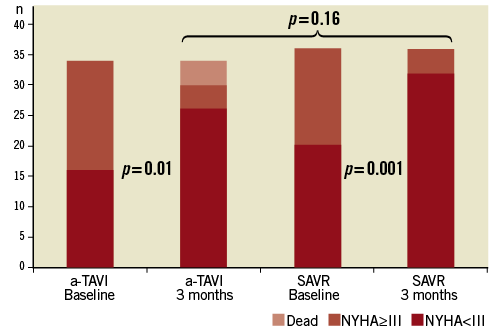
Figure 3. The New York Heart Association (NYHA) functional class at baseline and three months after transapical transcatheter aortic valve implantation (a-TAVI) vs. surgical aortic valve replacement (SAVR).
Discussion
The STACCATO trial was prematurely terminated because of an overall excess of adverse events in transcatheter treated patients in comparison with patients receiving SAVR. The adverse events included significant in-lab complications (e.g., aortic rupture, THV embolisation and THV blockage of the left coronary artery), and cases of stroke and significant paravalvular leakage. This register of complications contrasted to a remarkably low event rate in the surgical group.
We planned and organised the randomised STACCATO trial because we felt a need for controlled trials assessing this new and promising treatment modality in surgically lower-risk patients. We selected the a-TAVI procedure, because we had experienced this procedure as safe, predictable and associated with excellent results in surgically high-risk patients.15
The results of our trial must be interpreted with caution, because of the early termination, and because of the small number of patients included; only one third of what was planned. Therefore, it must be borne in mind that the excess of adverse events in the a-TAVI group may be a play of chance. Nevertheless, we would focus on three important issues that may need attention in TAVI; possible device-related stroke, paravalvular leakage and perioperative coronary artery occlusion.
We saw four cases of stroke in the trial. All strokes were thromboembolic. There was a perioperative major stroke in one SAVR patient. In the a-TAVI group, there were two major strokes resulting in severe disability and death, and one patient with transient ischaemic attack. The major strokes in the a-TAVI group occurred 2-4 weeks after the index procedure, and might be embolic and device-related. The same problem was described in the TAVI patients of The PARTNER Cohort A trial comparing TAVI with SAVR in surgically high operative risk patients.3 In the present trial, as in the PARTNER trial, the events occurred during the recommended period of antithrombotic therapy with aspirin and clopidogrel.
Two a-TAVI patients were re-operated because of severe paravalvular leakage due to undersized valves. In addition, we found considerably more leakage by echocardiography in a-TAVI patients than in the SAVR group. Leakage is a well-known finding after TAVI in registries and in the randomised PARTNER trial.3,15,17 However, the long-term clinical significance of the finding is unknown. We recognised the TAVI leakage problem as a sizing problem that might be solved by optimising preoperative valve annulus assessment using multislice computed tomography (MSCT),18 and in a general upsizing of the valve implants. Here, the introduction of a 29 mm valve may become an important measure. It is likely that the TOE long-axis view of the aortic root that was used underestimates the true annulus size because of the larger transversal dimensions of an oval annulus. Unfortunately, the transversal diameter is difficult to visualise by means of TOE. During the study period, MSCT of the aortic annulus was not part of the pre-procedure routine in our TAVI programme, as it is now. Also, the complicating rupture of the aortic annulus in one of our early a-TAVI patients was caused by an inaccurate pre-procedure annulus sizing. Here, it may be emphasised that the number of valve sizes during SAVR is much greater than in TAVI, giving the surgeon better opportunities to select an appropriately sized valve.
One patient deteriorated haemodynamically immediately after valve insertion with severe impairment of the left ventricular function and electrocardiographic ST-segment elevation, because of impaired flow in the left coronary artery. The patient had a technically successful acute coronary bypass operation and aortic valve replacement, but died because of liver rupture caused by external cardiac massage. Again, this complication might have been avoided by improved pre-operative assessment of the valve to coronary artery relationship using MSCT.
The adverse events in our a-TAVI patients were not related to or a result of the apical approach, and might equally well have occurred after femoral access TAVI procedures. It is remarkable that the a-TAVI patients did not differ from the SAVR group regarding physical and mental functional scores. Also, the length of hospital stay was similar in the two groups. The discomfort caused by the thoracotomy used in a-TAVI procedures may contribute to these findings.
The study was planned as a multicentre study, but at study termination, only two centres were actively included. Therefore, results are dependent on the treatment quality of the two participating centres. The SAVR results were excellent, but we cannot exclude that our results might have changed with participation of more centres. The TAVI experience of the participating centres was reasonably high and our previously published results15 from a high-risk TAVI cohort was similar to other registry reports4,5,7,10,19. However, the a-TAVI device success rate of 79% in the present study is lower than in the Partner Trial, and also lower than in the a-TAVI cohort of inoperable patients from our own institution, where we had a device success of 91%.15
According to the logistic EuroSCORE and the STS score, the risk profile of our patients was considerably more favourable than in the PARTNER Cohort A trial3, where TAVI compared well with SAVR.
Conclusion
Given the limitations of a small prematurely terminated study, our results suggest that a-TAVI in its present form may be associated with complication and device success rates in low-risk patients similar or even inferior to those found in high-risk patients with aortic valve stenosis. This will probably change in the near future with improved catheter-based devices and better pre-procedural assessment.
Acknowledgement
Data safety monitoring board
Professor Truls Myrmel, Department of Cardiac Surgery, University of Northern Norway, Tromsoe, Norway; Professor Dag Sørlie, Department of Cardiac Surgery, University of Northern Norway, Tromsoe, Norway; associate professor Terje Steigen, Department of Cardiology, University of Northern Norway, Tromsoe, Norway.
Independent endpoint committee
Associate professor Jens F. Lassen, Department of Cardiology, Aarhus University Hospital, Denmark; Associate professor Claus Ziegler Simonsen, Department of Neurology, Aarhus University Hospital, Denmark.
Conflict of interest statement
K.E. Klaaborg and L. Thuesen are part-time physician proctors for Edwards Lifesciences. The other authors have no conflict of interest to declare. The present study was conducted without any relationship to industry.

