Abstract
Aims: It is unclear whether transcatheter aortic valve implantation (TAVI) addresses an unmet clinical need for those currently rejected for surgical aortic valve replacement (SAVR) and whether there is a subgroup of high-risk patients benefiting more from TAVI compared to SAVR. In this two-centre, prospective cohort study, we compared baseline characteristics and 30-day mortality between TAVI and SAVR in consecutive patients undergoing invasive treatment for aortic stenosis.
Methods and results: We pre-specified different adjustment methods to examine the effect of TAVI as compared with SAVR on overall 30-day mortality: crude univariable logistic regression analysis, multivariable analysis adjusted for baseline characteristics, analysis adjusted for propensity scores, propensity score matched analysis, and weighted analysis using the inverse probability of treatment (IPT) as weights. A total of 1,122 patients were included in the study: 114 undergoing TAVI and 1,008 patients undergoing SAVR. The crude mortality rate was greater in the TAVI group (9.6% vs. 2.3%) yielding an odds ratio [OR] of 4.57 (95%-CI 2.17-9.65). Compared to patients undergoing SAVR, patients with TAVI were older, more likely to be in NYHA class III and IV, and had a considerably higher logistic EuroSCORE and more comorbid conditions. Adjusted OR depended on the method used to control for confounding and ranged from 0.60 (0.11-3.36) to 7.57 (0.91-63.0). We examined the distribution of propensity scores and found scores to overlap sufficiently only in a narrow range. In patients with sufficient overlap of propensity scores, adjusted OR ranged from 0.35 (0.04-2.72) to 3.17 (0.31 to 31.9). In patients with insufficient overlap, we consistently found increased odds of death associated with TAVI compared with SAVR irrespective of the method used to control confounding, with adjusted OR ranging from 5.88 (0.67-51.8) to 25.7 (0.88-750). Approximately one third of patients undergoing TAVI were found to be potentially eligible for a randomised comparison of TAVI versus SAVR.
Conclusions: Both measured and unmeasured confounding limit the conclusions that can be drawn from observational comparisons of TAVI versus SAVR. Our study indicates that TAVI could be associated with either substantial benefits or harms. Randomised comparisons of TAVI versus SAVR are warranted.
Introduction
Transcatheter aortic valve implantation (TAVI) is currently restricted to patients with aortic stenosis in whom surgical aortic valve replacement (SAVR) would be associated with a high or prohibitive risk of morbidity and/or mortality. The aim of TAVI is to provide a minimally invasive treatment that is at least as effective and associated with less morbidity and mortality compared to conventional valve surgery in high-risk patients. It is unclear whether TAVI addresses an unmet clinical need for those currently rejected for SAVR and whether there is a subgroup of high-risk SAVR patients who can benefit more from TAVI than SAVR.
Contemporary studies indicate that the 30-day mortality rate following TAVI is 8% to 12%1-3. Following SAVR, the 30-day mortality rate in high-risk patient subsets was reported to be between 4.6% to 13.5% for octogenarians4-11 and 6% to 33% for patients with left ventricular dysfunction12,13. To our knowledge, direct comparisons between TAVI and SAVR are not yet available. In this two-centre, prospective cohort study, we set out to compare the characteristics at baseline and 30-day mortality rates between TAVI and SAVR in consecutive patients undergoing invasive treatment for aortic stenosis.
Patients and methods
Between January 1st, 2006 and December 31st, 2008, we prospectively enrolled consecutive patients with aortic stenosis who underwent invasive treatment for aortic stenosis at the Erasmus Medical Center, Rotterdam, The Netherlands and Bern University Hospital, Bern, Switzerland. The study complies with the Declaration of Helsinki and was approved by the local Research Ethics Committees. All patients provided written informed consent.
Patients and interventions
Patients were included if they underwent TAVI or SAVR for the treatment of aortic stenosis at one of the two institutions. Patients undergoing invasive treatment who had a primary diagnosis of aortic regurgitation, multiple valve interventions, or concomitant aortic root reconstruction were excluded. Contraindications for TAVI or SAVR typically included sepsis, bleeding diathesis or coagulopathy, any condition considered a contraindication to extracorporeal assistance, or estimated life expectancy of less than one year.
TAVI was performed using the third generation CoreValve ReValving System (Medtronic, Minneapolis, MN, USA). For patients to undergo TAVI, an interventional cardiologist and cardiac surgeon had to agree that conventional open-heart surgery would be associated with excessive morbidity and mortality. Details of the device, and technical aspects of the procedure have been previously published14. The procedure was performed with the patient under general anaesthesia or with local anaesthesia and mild sedation depending on patient characteristics. Vascular access was obtained percutaneously using a «pre-closing» device (10 Fr Prostar XL) or by surgical cut-down14. Coronary revascularisation was performed if deemed necessary prior to or during the index procedure using percutaneous coronary intervention (PCI)15.
The choice of implant used and the technique for SAVR was left to the discretion of the treating cardiac surgeon. The procedure was performed with the patient under general anaesthesia using cardiopulmonary bypass. Coronary revascularisation was performed if deemed necessary during the index procedure using coronary artery bypass graft surgery (CABG).
Baseline characteristics
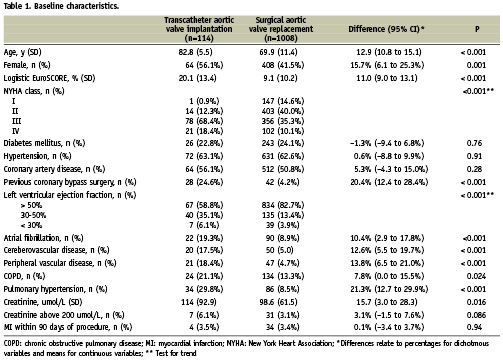
The selection of baseline characteristics was based on expert opinion and a review of the literature. We considered patient age (years), sex, logistic EuroSCORE16, New York Heart Association class, ejection fraction (>50%, 30-50%, or <30%), creatinine concentration (umol/L), a history of coronary artery disease, myocardial infarction in the previous 90 days, CABG, atrial fibrillation, peripheral vascular disease, cerebrovascular disease, pulmonary hypertension, and COPD.
Procedural characteristics
We recorded whether the procedure was an emergency measure, defined as a procedure carried out on referral before the beginning of the next working day16 and whether there was a concomitant coronary revascularisation using PCI in patients undergoing TAVI and CABG in patients undergoing SAVR.
Primary outcome and definitions
The primary outcome was all-cause mortality within 30 days17. Patients were actively followed-up until 30 days after the index procedure and their vital status was confirmed by outpatient clinical visit, telephone visit, review of medical records or through civil registries.
Statistical methods
We compared baseline characteristics between patients who had undergone TAVI and SAVR using a X2 test for categorical variables and an unpaired t test for continuous variables, and used uni- and multivariable logistic regression models to determine the association of baseline characteristics with 30-day mortality. The propensity scores of SAVR patients were estimated using a probit model with all baseline characteristics as described above as independent variables. The propensity score is the probability that a patient would have been treated with SAVR given that patient’s observed baseline characteristics. Patients with the same propensity score must have the same distribution of baseline characteristics. To determine whether this assumption of balanced baseline characteristics was satisfied, we used standard algorithms implemented in the statistical package. Then, we calculated the c-statistic (the area under the receiver operating characteristics curve for the ability of propensity scores to predict actual treatment) as an estimate of the adequacy of the model.
To ensure the quality of the matching using propensity scores, we applied the common support assumption. The common support assumption indicates that patients with the same propensity score have a non-zero probability of receiving both, TAVI or SAVR18. If probability densities are too low in one of the groups, there is insufficient overlap of propensity scores and the assumption of common support is unlikely to be satisfied. To address this, we estimated the probability densities of propensity scores of patients in the TAVI and SAVR group using Epanechnikov kernel probability density estimates based on propensity score increments of 0.02519. We pre-specified a probability density of >0.5 to ensure sufficient overlap of propensity scores20. Below this pre-specified probability density (established at a propensity score <0.675), patients who had undergone TAVI were considered unlikely to be comparable with patients of the same propensity score who had undergone SAVR.
We used logistic regression models to compare 30-day mortality between groups. We pre-specified the following seven approaches for analysis: (1) crude univariable analysis; (2) multivariable analysis adjusted for all baseline characteristics described above; (3) bivariable analysis adjusted for the propensity score as a linear term21; (4) multivariable analysis adjusted for the propensity score and for all baseline characteristics; (5) univariable analysis after caliper matching on the propensity score in a range of ±0.0522; (6) weighted univariable analysis using the inverse probability of treatment (IPT) as weights23,24; (7) IPT weighted multivariable analysis adjusted for all baseline characteristics. The IPT weighted analyses used the inverse of the propensity score as weights in SAVR patients and the inverse of 1 minus the propensity score in TAVI patients. All analyses were based on logistic regression models and were performed in the overall dataset and stratified according to presence or absence of sufficient overlap of propensity scores (propensity score <0.675 versus ≥0.675). To determine whether there was an interaction between estimated odds ratios of death and overlap of propensity scores, we performed a formal interaction test based on z-scores. Then, we performed a sensitivity analysis (not pre-specified), additionally adjusting IPT weighted analyses restricted to patients with propensity scores ≥0.675 for the procedural characteristics described above. Finally, we considered patients undergoing TAVI with propensity scores ≥0.675 to be potentially eligible for a randomised comparison of TAVI and SAVR and compared pre-treatment characteristics of these patients with characteristics of patients undergoing TAVI with propensity scores <0.675 who were deemed ineligible for a randomised comparison with SAVR. All p-values are two-sided. Statistical analyses were performed using Stata Version 10 (Stata Corporation, College Station, TX, USA).
Results
Between January 2006 and December 2008, 1,633 consecutive patients underwent invasive treatment for aortic stenosis using TAVI or SAVR. After exclusion of 508 patients because of a primary diagnosis of aortic regurgitation, multiple valve surgery, or concomitant aortic root surgery, 1,122 patients (1,122 procedures) were included in the study: 114 undergoing TAVI (52 Bern and 62 Rotterdam) and 1,008 patients undergoing SAVR (645 Bern and 363 Rotterdam). No patient was lost to follow-up at 30 days after the index procedure.
Baseline characteristics of patients
Baseline characteristics of the TAVI and SAVR patients are summarised in Table 1. Compared to patients undergoing SAVR, patients with TAVI were clearly older, more often female, more likely to be in NYHA class III and IV, had a considerably higher logistic EuroSCORE and more comorbid conditions, including previous coronary artery bypass surgery, left ventricular dysfunction, atrial fibrillation, cerebral and peripheral vascular disease, chronic obstructive pulmonary disease, and pulmonary hypertension.
Procedural characteristics
The procedure was performed as an emergency measure in two TAVI patients (1.8%) and 43 SAVR patients (4.3%) (difference –2.4%, 95% confidence interval [CI] –5.1 to 0.3%). Concomitant revascularisation was performed in 10 TAVI patients (8.8%) and 436 SAVR patients (43.5%) (difference -34.7%, 95% CI –40.7 to –28.7%). An additional 10 TAVI patients (8.8%) had coronary revascularisation within four weeks prior to valve implantation as a staged procedure.
Association of baseline characteristics with 30-day mortality
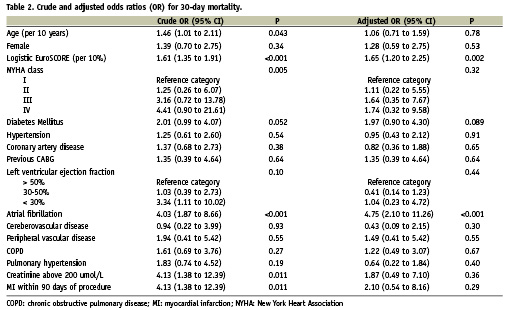
Table 2 presents the crude and adjusted odds ratios for 30-day mortality. Univariable analysis revealed that age, logistic EuroSCORE, previous coronary artery bypass, atrial fibrillation, renal failure (creatinine >200 µmol/L) and myocardial infarction within 90 days of the procedure were associated with 30-day mortality at p <0.05; a trend was noted for diabetes mellitus (p=0.051). In multivariable analysis, only logistic EuroSCORE and atrial fibrillation were associated with mortality at p<0.05, and a trend remained for diabetes mellitus (p=0.089).
Propensity scores
The model used to estimate propensity scores yielded a c-statistic of 0.93. Figure 1 shows the distribution of propensity scores. The mean propensity score to receive SAVR was 0.53±0.28 for patients undergoing TAVI compared with 0.94±0.12 for patients actually undergoing SAVR. The distribution was symmetrical around 0.5 for the TAVI group, but clearly shifted towards 1 for the SAVR group. Based on the common support assumption, the area of sufficient overlap of propensity scores was small (Figure 1, white area to the right): in patients undergoing SAVR, the probability density fell definitely below the pre-specified value of 0.5 at a propensity score of 0.675. In patients undergoing TAVI, 39 patients (34.2%) were above and 75 (65.8%) were below the cut-off of 0.675. In patients undergoing SAVR, 957 patients (94.9%) were above and 51 (5.1%) were below the cut-off. Using simply the propensity scores with a calliper matching range of 0.05, 81 patients undergoing TAVI (71.1%) could each be matched to a patient in the SAVR group. Of these 81 pairs of patients, 39 were above and 42 pairs were below the cut-off of 0.675.
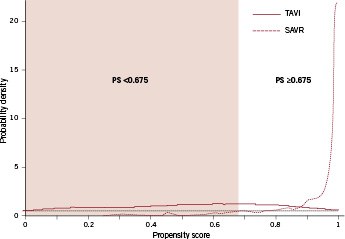
Figure 1. Probability density function of the propensity score for 114patients undergoing TAVI (red solid line) and 1,008 patients undergoingSAVR (red dotted line). The black dotted line represents the pre-specified probability density of >0.5; propensity score matching above this probability density ensures sufficient overlap of propensity scores. The white area indicates the range of propensityscores with sufficient overlap (propensity score ≥0.675) and identifies patients potentially eligible for a randomised comparison of TAVI and SAVR. The coloured area indicates the range of propensity scores with insufficient overlap and identifies patients likely to be ineligible for a randomised comparison.
Impact of different approaches used for control of confounding on mortality estimates in overall dataset
Figure 2 presents the results of the stepwise procedure to control for confounding in the analysis of the overall dataset. Within 30 days of the index procedure, 11 patients (9.6%) who had undergone TAVI and 23 patients (2.3%) who had undergone SAVR died (crude odds ratio [OR] 4.57, 95% CI, 2.17 to 9.65, Figure 2). The estimated odds ratios decreased only slightly after multivariable adjustment and propensity score adjustment. This suggested an approximately threefold increase in the odds of dying with TAVI as compared with SAVR. The estimated odds ratio increased in the analysis of propensity score matched patients to 7.57 (95% CI 0.91 to 63.0). Conversely, when the IPT weights were used, we found the estimated odds ratios decreased to 0.60 in the univariable analysis (95% CI 0.11 to 3.36) and to 1.25 in the multivariable analysis (95% CI 0.42 to 3.72).
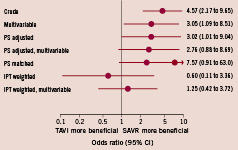
Figure 2. Impact of different approaches used to control for confounding on mortality estimates. The propensity score matched analysis was based on 162 patients (81 pairs), all other analyses were based on 1,122 patients. PS: propensity score; IPT: inverse probability of treatment.
Analyses stratified according to overlap of propensity scores
Figure 3 shows the results of the analyses stratified according to overlap of propensity scores. Among patients with propensity scores ≥ 0.675 and hence sufficient overlap of propensity scores, three out of 39 patients (8.0%) who had undergone TAVI and 22 out of 957 patients (2.8%) who had undergone SAVR died (crude OR 3.54, 95% CI 1.01 to 12.3, Figure 2, top left). The estimated odds ratios decreased after multivariable adjustment (OR 2.72), propensity score adjustment (1.87), but not after propensity score matching (3.17). The 95% CIs, however, largely overlapped the line of null effect at 1. When using the IPT weights, we found the estimated odds ratios to decrease below 1 in both the univariable (OR 0.35, 95% CI 0.04 to 2.72) and multivariable analyses (OR 0.87, 95% CI 0.19 to 4.04). We found similar results after adjusting the IPT weighted analyses for procedural characteristics (emergency measure and concomitant revascularisation); the estimated odds ratios in patients with propensity scores ≥ 0.675 was 0.73 (95% CI 0.07 to 7.70) after the weighted univariable analysis and 1.63 (95% CI 0.22 to 12.1) after the weighted multivariable analysis.
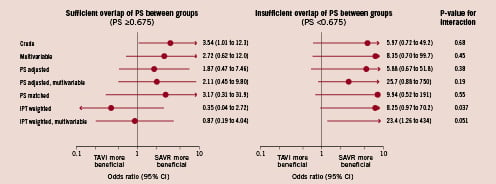
Figure 3. Analyses stratified according to overlap of propensity scores (propensity scores ≥0.675 versus <0.675). Among patients with sufficient overlap (left), the propensity score matched analysis was based on 78 patients (39 pairs), all other analyses were based on 996 patients. Among patients with insufficient overlap (right), the propensity score matched analysis was based on 84 patients (42 pairs), all other analyses were based on 126 patients
Among patients with insufficient overlap of propensity scores (propensity scores <0.675), eight out of 75 patients (10.7%) who had undergone TAVI and one out of 51 patients (2.0%) who had undergone SAVR died (crude OR 5.97, 95% CI 0.72 to 49.2, Figure 2, top right). The estimated odds ratios were between 5.88 and 25.7 depending on the approach used to control for confounding. Tests of interaction between estimated odds ratios and overlap were positive at the conventional significance level for univariable IPT weighted analyses (p=0.037) and of borderline significance for multivariable IPT weighted analyses (p=0.051, Figure 3).
Patients potentially eligible for a randomised trial
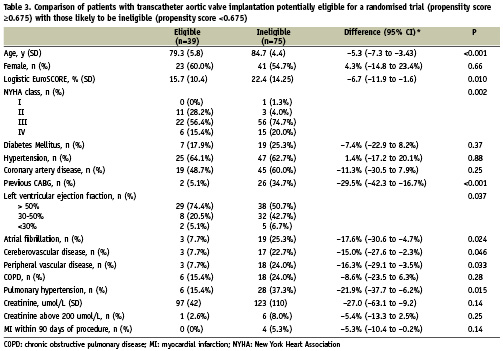
Table 3 presents the baseline characteristics of TAVI patients who had propensity scores ≥0.675 and were considered to be potentially eligible for a randomised comparison of TAVI and SAVR compared with the characteristics of TAVI patients with propensity scores <0.675 deemed to be ineligible. Patients eligible for a randomised trial were younger, had less severe symptoms as measured by the NYHA class, had lower logistic EuroSCORES, and were less likely to report a history of previous coronary artery bypass, atrial fibrillation, stroke, peripheral vascular disease, or pulmonary hypertension.
Discussion
In this prospective cohort study we found that both measured and unmeasured confounding complicated the observational comparison of 30-day mortality of TAVI versus SAVR. Compared to SAVR patients, TAVI patients were clearly older, more often female, more likely to be in NYHA class III and IV, had a considerably higher logistic EuroSCORE and had more comorbid conditions, including previous coronary artery bypass surgery, left ventricular dysfunction, atrial fibrillation, cerebral and peripheral vascular disease, chronic obstructive pulmonary disease, and pulmonary hypertension. When naively analysing the overall dataset of 1,122 consecutive patients included in our study, we found a 4.5-fold increase in the odds of death associated with TAVI as compared with SAVR. The odds ratio, however, ranged from 0.6 to 7.57 depending on the method used to control for confounding. These results indicate that TAVI may be associated with potential benefits or harm. Given the fact that TAVI was performed in either high risk or inoperable patients, analysis of the overall dataset may suffer from confounding by indication. For the inoperable patients, the relevant comparison would have been medically managed patients. In order to minimise confounding by indication, we identified patients from the TAVI and SAVR group who, in addition to having similar propensity scores, would also be appropriate for either type of treatment.
Below a propensity score cut-off of 0.675, it was deemed unlikely that two patients with the same propensity score, one who actually received TAVI, the other SAVR, had indeed identical probabilities to undergo SAVR. Two-thirds of patients undergoing TAVI, but only 5% of those undergoing SAVR had scores below this cut-off. Restricting the analysis to patients with sufficient overlap of propensity scores ≥ 0.675, we found little evidence for an excess mortality in TAVI patients. On the other hand, the estimated odds ratios greatly increased between 5.9 and 25.7 in those patients with insufficient overlap of propensity scores. These results suggest that unmeasured confounding factors complicated the observational comparisons of TAVI versus SAVR, albeit more in patients with insufficient than sufficient overlap of propensity scores. Examples of unmeasured confounding variables that could be associated with both the selection of treatment and prognosis include porcelain aorta, history of mediastinal radiation, or frailty. In addition, we were unable to record the clinical judgment of the treating physicians, which may be a relevant proxy for prognosis. In light of the high probability of residual confounding complicating any observational study in this field, randomised controlled trials comparing TAVI and SAVR are clearly warranted.
Through our examination of propensity score distributions, we characterised a subgroup of TAVI patients likely to be eligible for a randomised trial (Table 3). In our institutions, the following criteria are used to guide the selection of patients for TAVI: age ≥75 years, a logistic EuroSCORE ≥15%, and/or age ≥65 years associated with severe limiting comorbid conditions. Our study indicates that approximately one-third of patients selected for TAVI (based on these criteria) would be eligible for a randomised comparison of TAVI and SAVR. These patients were younger, had lower logistic EuroScores, and were less likely to report a history of previous coronary artery bypass, atrial fibrillation, stroke, peripheral vascular disease, or pulmonary hypertension than potentially ineligible TAVI patients. Based on the exploratory nature of our study, we cannot provide a firm basis for the identification of patients eligible for such a trial.
The small number of patients undergoing TAVI and the small number of accumulated deaths was a limitation of this study. The multivariable analyses was difficult particularly when numbers were cut down through stratification of analyses according to overlap of propensity scores. The stratified multivariable analyses should therefore be interpreted with discretion. The small number of patients and events also meant that the most reliable analyses, which were restricted to patients with sufficient overlap of propensity scores, were imprecise. Whereas we have little evidence to suggest that TAVI is associated with increased mortality as compared with SAVR, the 95% CIs from these analyses are wide and compatible with either substantial benefits or harms of this new intervention.
The strengths of this study include the following: To our knowledge, this is a unique endeavour to compare the characteristics and outcomes of patients with aortic stenosis undergoing TAVI and SAVR. Secondly, an interdisciplinary team of cardiologists, cardiac surgeons, statisticians and clinical epidemiologists performed a thorough examination of the potential for measured and unmeasured confounding. Thirdly, we had complete follow-up within the first 30 days of the index procedure.
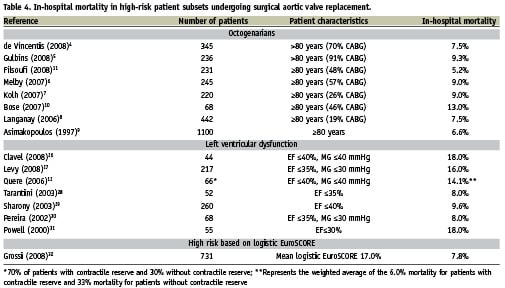
The unadjusted 30-day mortality rates were 9.6% and 2.3%, for TAVI and SAVR groups, respectively. The results for the TAVI group compare favourably with contemporary outcome reports for the CoreValve ReValving System1-3. The 30-day mortality rate for the SAVR group was more than two times lower than the unadjusted mortality rate of 5.7% reported by Rankin et al for 216,245 patients undergoing single aortic valve replacement25. The TAVI group was mainly comprised of octogenarians and approximately 40% of patients had left ventricular dysfunction (EF <50%). Table 4 presents the in-hospital mortality rates of high-risk patients undergoing SAVR. The 30-day mortality rate of 9.6% observed in TAVI patients in our study is encouraging in light of the reported in-hospital mortality rates of selected octogenarians (4.6% to 13.5%)4-11, patients with left ventricular dysfunction (6% to 33%)12,13,26-31 and patients with high logistic EuroSCORES (7.8%) undergoing surgical aortic valve replacement32.
While surgical heart valve replacement remains the standard of care, several studies have demonstrated that 30% to 60% of patients with symptomatic severe aortic valve stenosis are denied or not referred for surgery33-36. Patients who are denied or not referred for surgical aortic valve replacement are typically older, have moderate impairment of ejection fraction and more non-cardiac co-morbidities than patients undergoing valve surgery33-36. TAVI meets an unmet clinical need in these patients and may therefore be considered a breakthrough technology37.
Conclusions
Both measured and unmeasured confounding limits the conclusions that can be drawn from observational comparisons of TAVI versus SAVR. Our study indicates that TAVI could be associated with either substantial benefits or harms. Randomised comparisons of TAVI and SAVR are warranted to provide evidence-based medicine and legitimise the use of TAVI in the eyes of the medical community, non-invasive cardiologist, cardiac surgeon, and health authorities. In addition, these trials will play a crucial role in reimbursement policies.
Acknowledgements
We are grateful to Jonathan Sterne and Marcel Zwahlen for helpful discussions related to the analytical strategies used in this study. The analysis was funded by intramural grants provided by CTU Bern, Bern University Hospital, the Institute of Social and Preventive Medicine, University of Bern, and the Swiss National Science Foundation to Peter Jüni, a PROSPER fellow funded by the Swiss National Science Foundation. CTU Bern is supported by the Swiss National Science Foundation.

