Abstract
Aims: To review the outcomes of studies and the safety of newer transcatheter aortic valves (THV).
Methods and results: All studies reporting on second-generation THV were identified and pooled using the systematic review guidelines. Twenty-four reports on 1,708 patients and eight THV were included in the analysis. The pooled 30-day event rate for mortality after transcatheter aortic valve implantation (TAVI) was 5.7% (95% CI: 4.0-7.8), myocardial infarction (MI) was 1.7% (95% CI: 1.1-2.6), stage 3 acute kidney injury (AKI) was 3.4% (95% CI: 2.0-5.6), life-threatening bleeding was 5.1% (95% CI: 3.3-7.8), major vascular complications was 4.9% (95% CI: 3.5-6.6%), major bleeding was 10.5% (95% CI: 5.1-20.4), major stroke was 2.4% (95% CI: 1.7-3.4), permanent pacemaker utilisation was 13.5% (95% CI: 10.8-16.9), and coronary obstruction was 1.2% (95% CI: 0.6%-2.4%). Moderate or severe aortic insufficiency (AI) after TAVI was 4.2% (95% CI: 2.0-8.5). The pooled 30-day mean gradient and effective orifice area (EOA) were 11.63 mmHg (95% CI: 10.19-13.07) and 1.60 cm2 (95% CI: 1.5-1.7), respectively. All estimates compare favourably to events reported for first-generation valves.
Conclusions: Our findings suggest that the new THV have a low risk of TAVI-related short-term complications.
Abbreviations
CE: Conformité Européenne (European Conformity)
CI: confidence interval
DFM: Direct Flow Medical valve
DISCOVER: A registry to evaluate the Direct Flow Medical transcatheter aortic valve system
EHJ: European Heart Journal
EJCTS: European Journal of Cardio-Thoracic Surgery
FIM: first-in-man
GARY: German Aortic Valve Registry
ICI: innovations in cardiovascular interventions
IDE: investigational device evaluation
JACC: Journal of American College of Cardiology
JTCS: Journal of Thoracic and Cardiovascular Surgery
JUPITER: long-term safety and performance of the JenaValve trial
MC: multicentre
N: number of patients
NA: not available
PARTNER: Placement of AoRTic TraNscathetER Valves trial
Reg: registry
REPRISE: REpositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus™ Valve System study
SAVI: Symetis ACURATE TA™ valve implantation registry
TA: transapical
TCT: transcatheter cardiovascular therapeutics
TF: transfemoral
VARC: Valve Academic Research Consortium
UK: United Kingdom
Introduction
Transcatheter aortic valve implantation (TAVI) has seen an exponential utilisation in high surgical risk patients due to impressive results in randomised and non-randomised trials1,2. The wealth of this experience is shared by the Medtronic CoreValve® (Medtronic, Minneapolis, MN, USA) and the Edwards SAPIEN valve (Edwards Lifesciences, Irvine, CA, USA). However, despite their good results, both valves have significant limitations which restrict the expansion of TAVI to the intermediate- and low-risk population1-6. Neither device is truly repositionable or retrievable, making deployment a “single-shot” procedure and technically demanding. Malpositioning of the aortic prosthesis is therefore not uncommon and has been implicated in several TAVI-related complications which include paravalvular leak, coronary obstruction, valve embolisation, atrioventricular conduction block, heart failure and mortality. Major vascular complications with attendant risk of renal failure and mortality are also of concern with these early devices for TAVI. Newer, second-generation transcatheter aortic valves have been developed to overcome the limitations of the Medtronic CoreValve and Edwards SAPIEN valve to improve deliverability, prevent paravalvular leak and to reposition or retrieve when necessary. A number of devices with these desirable features are being investigated and have undergone successful clinical evaluation with promising results. Data on the safety and efficacy of these devices are, however, limited by the small number of patients enrolled in each study7-15. We therefore performed this systematic review on the safety and efficacy of newer, second-generation TAVI devices to obtain more conclusive results.
Methods
STUDY SELECTION
We conducted this systematic review on the published literature of outcomes after TAVI using second-generation valves. The review was conducted as per the QUOROM16 (Quality of Reporting of Meta-Analysis) and MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines17. All valves (other than the Medtronic CoreValve/Edwards SAPIEN and SAPIEN XT or their precursors) which were designed to improve deliverability, sealing to prevent paravalvular leak and/or the ability to reposition or retrieve were considered to be second-generation valves. We reviewed the literature to identify all available valves, including the second-generation valves. A computerised search was then performed by two reviewers (G. Athappan and R.D. Gajulapalli) to identify all relevant studies using these second-generation valves published until January 2015 in the PubMed database or Major Conference proceedings. The following search terms were used: “TAVI”, “TAVR”, “Transcatheter Valve”, “Transapical Valve”, “ACURATE TA”, “ACURATE TF”, “ACURATE”, “Engager”, “JenaValve”, “Portico”, “Sadra Lotus” and “Direct Flow Medical Aortic Valve”, “CENTERA”, “CoreValve Evolut”, “HLT valve” and “SAPIEN 3”. Citations were screened at the title and abstract level and retrieved as a full report if they reported on safety outcomes with implantation of second-generation TAVI valves. Limiting the search parameters to the English language was subsequently applied. The full texts and bibliography of all potential articles were further reviewed in detail (G. Athappan) to seek additional relevant studies.
INCLUSION CRITERIA
Studies were included if the following criteria were satisfied: (a) TAVI was performed using second-generation valves, (b) the study included patients who had severe aortic stenosis of the native valve, and (c) a minimum of 10 patients were treated. When two similar studies were reported from the same institution or author, the most recent publication was included in the analysis.
EXCLUSION CRITERIA
Studies were excluded if any of the following criteria applied: (a) the outcome of interest was not clearly reported or was impossible to extract or calculate from the published results, or (b) the study involved valve-in-valve procedures.
DATA EXTRACTION
Relevant information was collected by G. Athappan/R.D. Gajulapalli and included, but was not limited to, first author, year and journal of publication, study design, inclusion/exclusion criteria, procedural outcomes, number of subjects included, subjects undergoing successful TAVI, type of device and approach used, study population demographics, follow-up time period and safety and efficacy outcomes.
STUDY ENDPOINTS
Safety endpoints: 30-day mortality, major stroke, life-threatening bleeding, major vascular complications, major bleeding, stage 3 acute kidney injury (AKI), coronary obstruction, MI, moderate or severe paravalvular AI and permanent pacemaker implantation. Valve efficacy endpoint: mean gradient and effective orifice area (EOA) at 30 days.
STATISTICAL ANALYSIS
DerSimonian and Laird’s random effects model was utilised to pool the estimates of 30-day safety and valve efficacy endpoints. Pooled event rates and 95% confidence intervals (95% CI) served as summary statistics. Reports with no reported events were excluded from the analysis. Statistical significance was set at p<0.05 (two-tailed). Heterogeneity was assessed by a Q-statistic and I2 test. Significant heterogeneity was considered present for I2≥50%. Subgroup analysis of studies reporting per VARC, core lab utilisation, transfemoral TAVI, transapical TAVI and post first-in-man (FIM) studies was also performed. The effect across subgroups (TF vs. TA) was compared using a Q-test for between-group heterogeneity. Data analysis was performed using Comprehensive Meta-Analysis Software Version 2 (BioStat, Englewood, NJ, USA)18.
Results
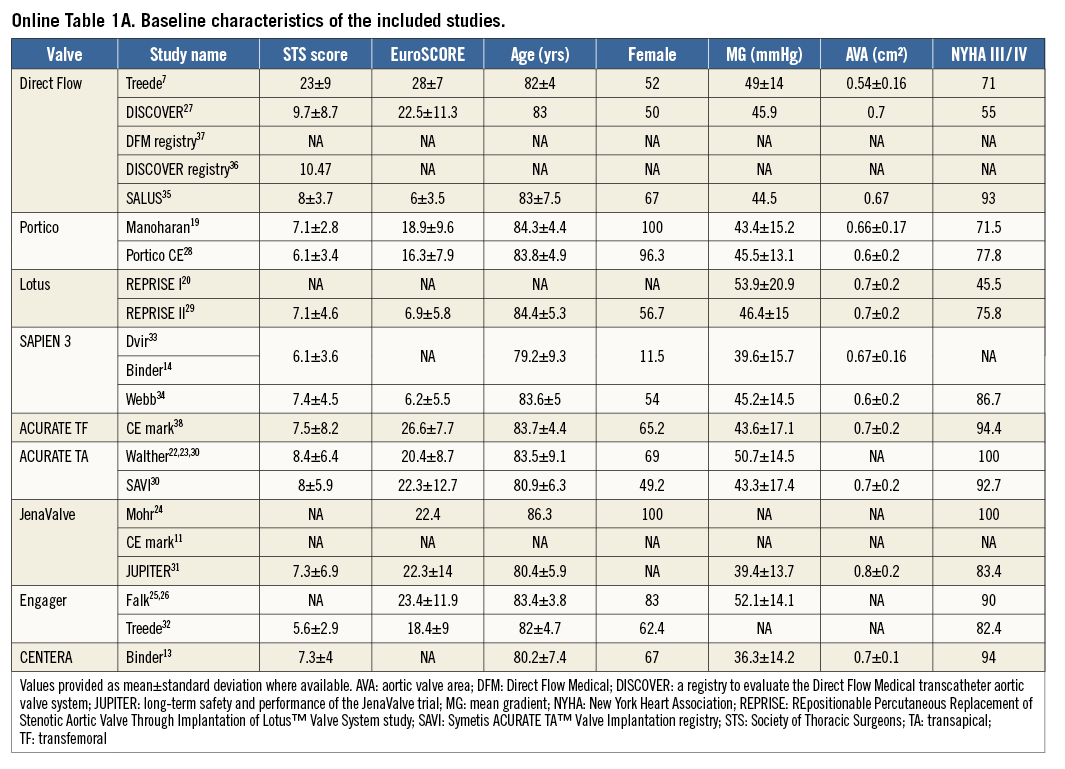
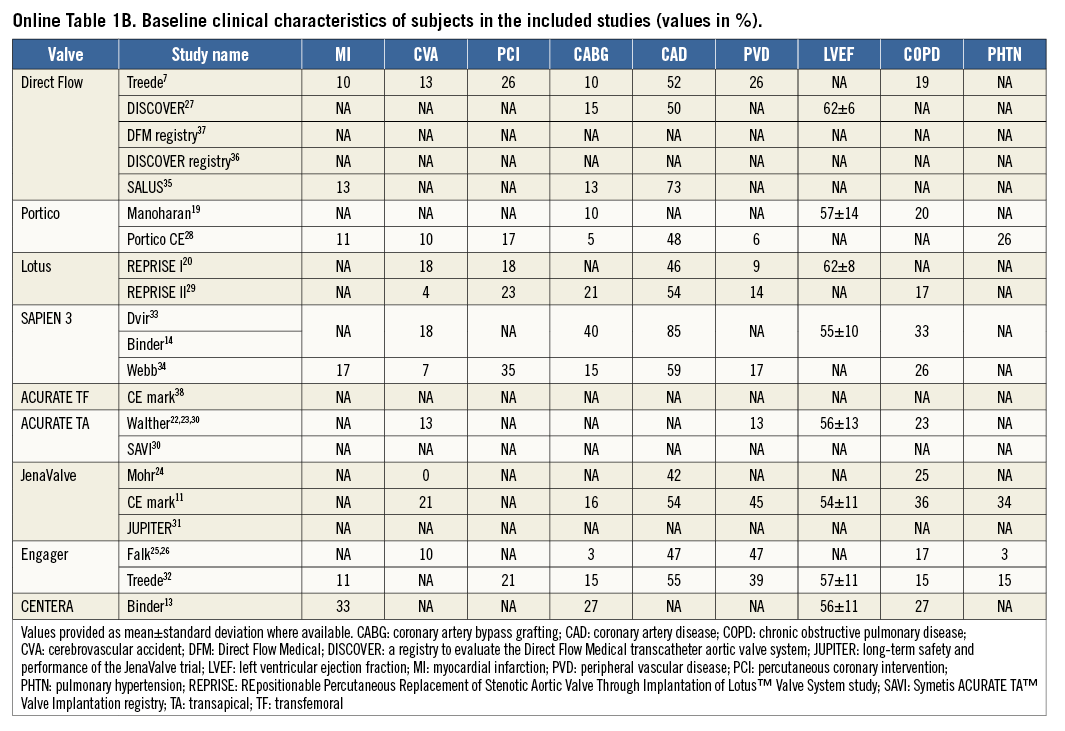
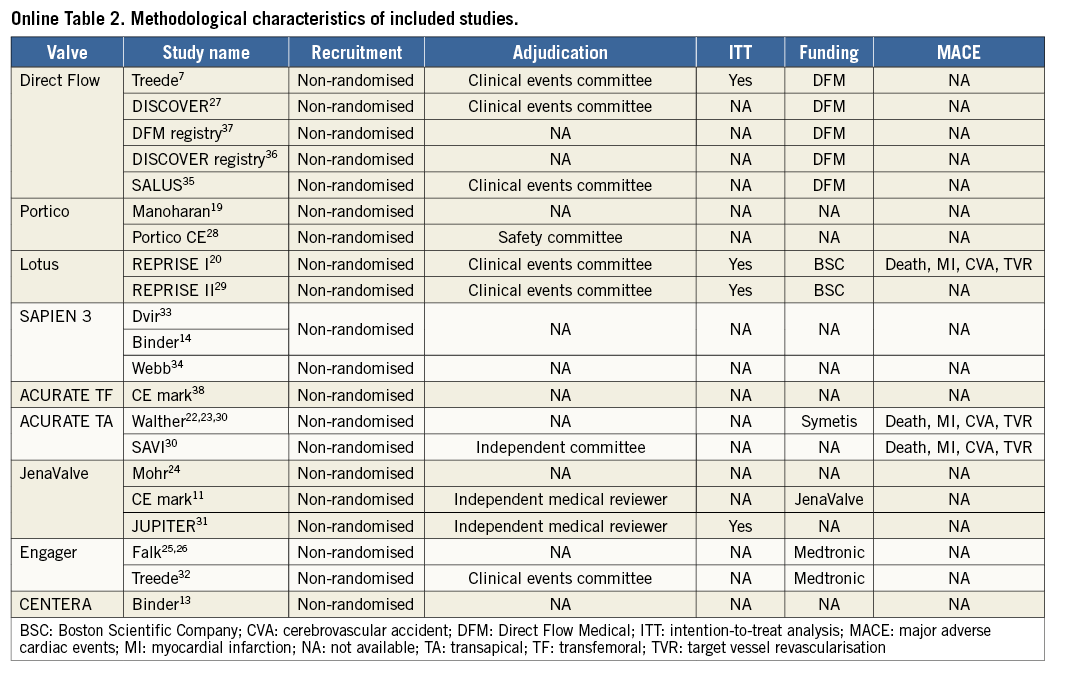
Using the search keywords, 5,734 reports were identified, of which 175 relevant publications were identified at the abstract and title levels. By applying the inclusion and exclusion criteria, 24 reports (Table 1) on 21 unique trials were selected for the meta-analysis7,11,13,14,19-38 (Figure 1). The study characteristics are provided in Online Table 1A & 1B whilst the methodological characteristics of studies are given in Online Table 2. These 24 unique trials were on eight unique second-generation valves that included 1,708 patients. Eight of these unique reports were “first-in-man” (FIM) studies7,13,14,19,20,22-26 on 236 patients (13.8%). In fifteen reports7,13,14,19-21,27-29,33-38 the transfemoral access was used (six unique valves, 891 patients: 52.2%), while the transapical access11,22-26,30-32,34 was used in nine (four unique valves, 817 patients: 47.8%). The ACURATE® valve (Symetis, Ecublens, Switzerland) and the SAPIEN 3 (Edwards Lifesciences) were available for both the transfemoral and the transapical approach.
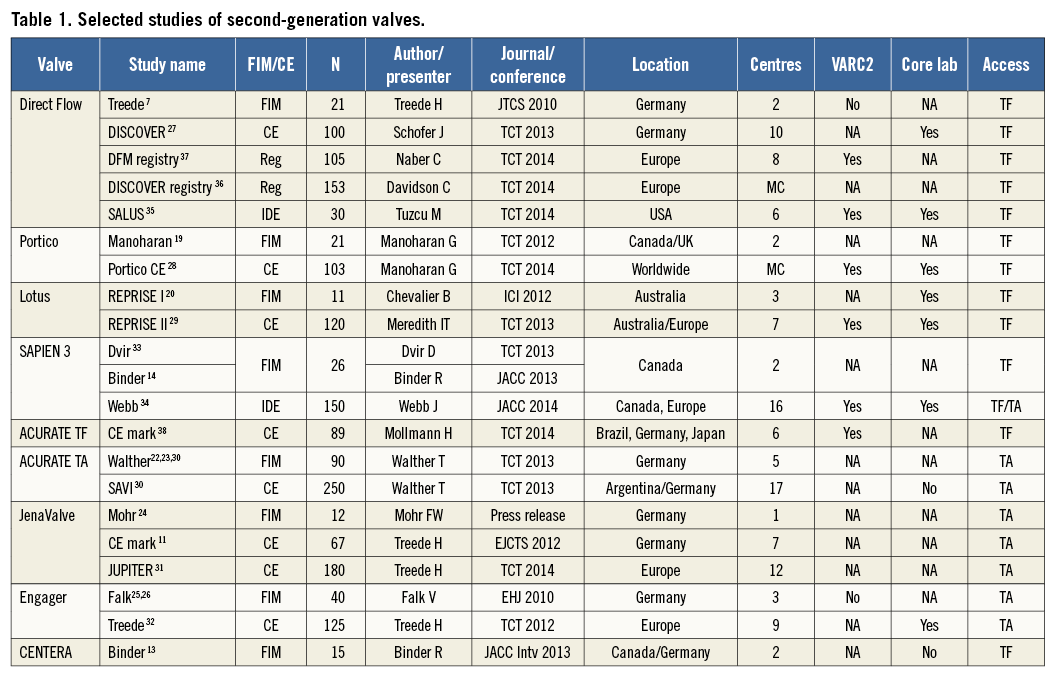
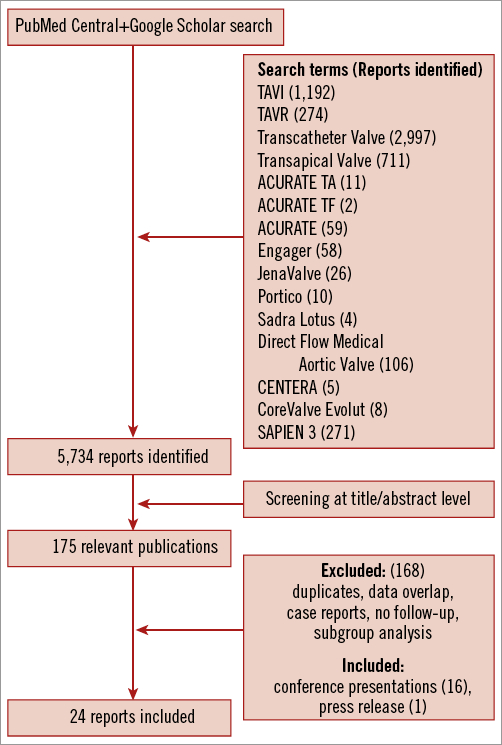
Figure 1. Flow chart showing selection of studies.
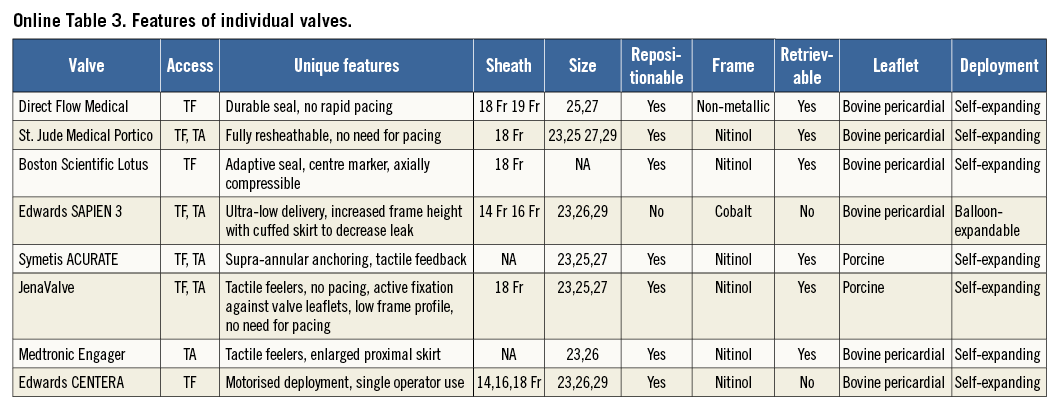
The types of implanted device were Direct Flow Medical® transcatheter aortic valve system (Direct Flow Medical Inc., Santa Rosa, CA, USA), 410 (24.0%); Lotus™ valve system (Boston Scientific, Marlborough, MA, USA), 131 (7.7%); Portico™ valve (St. Jude Medical, St. Paul, MN, USA), 124 (7.3%); SAPIEN 3 valve (Edwards Lifesciences), 76 (10.3%); JenaValve (JenaValve Technology, Munich, Germany), 258 (15.1%); ACURATE TF (Symetis), 89 (5.2%), ACURATE TA (Symetis), 340 (19.9%), CENTERA valve (Edwards Lifesciences), 15 (0.9%); and the Medtronic Engager™ valve (Medtronic), in 165 (9.7%) patients. Features of the individual valves are provided in Online Table 3. Reported device success ranged from 64.5% to 100%. The lowest device success was reported for the Direct Flow valve in the FIM study. Excluding FIM studies, the device’s success was in the range of 89.5-100%. Twenty patients (1.1%) required more than one valve. Surgical conversion to valve replacement was needed in 24 patients (1.4%). Data on valve retrieval7,27,29 and resheathing9,13,19,28,29 were available in three and four reports, respectively. In the above reports, retrieval (24 attempts, 24 successful) and resheathing (57 attempts/57 successful) were successful in 100% of cases when attempted. Mean effective orifice area (EOA) and gradient at baseline were 0.67±0.18 cm2 and 44.8±16.09 mmHg, respectively. Pooled post-TAVI mean EOA and gradient were 1.55 cm2 (95% CI: 1.43-1.67) and 10.85 mmHg (95% CI: 9.59-12.11), respectively.
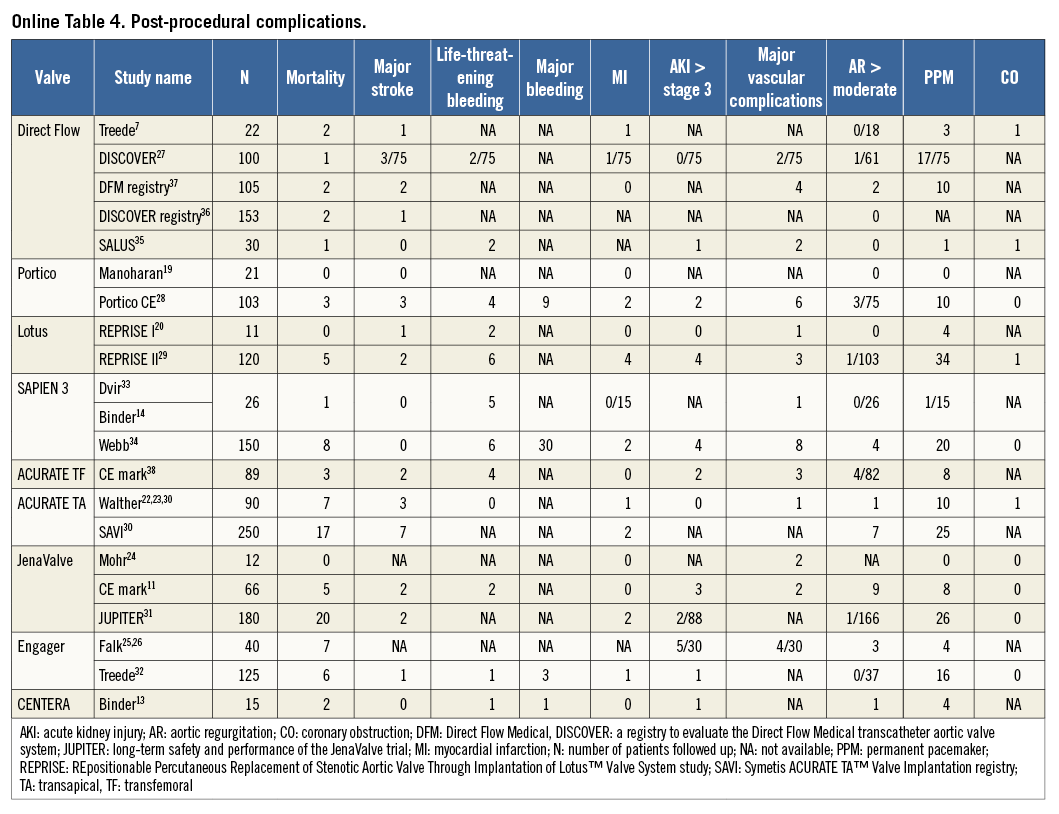
All-cause 30-day mortality rates were reported between 0% and 17.5%, with a pooled estimate rate of 5.7% (95% CI: 4.1%-7.8%, I2=48.53) (Figure 2). Myocardial infarction (MI) was reported as a complication of TAVR in 0% to 5.0% of studies, with a pooled estimate rate of 1.7% (95% CI: 1.1%-2.6%, I2=0) (Figure 3). Major strokes were reported from 0% to 9.1% and occurred at a pooled estimate rate of 2.4% (95% CI: 1.7%-3.4%, I2=0) (Figure 3).Coronary obstruction was seen in 1.2% (95% CI: 0.6%-2.4%, I2=0.0) (Figure 3). Acute kidney injury (AKI) stage 3 was seen in 0% to 16.7%, with a pooled estimate rate of 3.4% (95% CI: 2.0%-5.6%, I2=36.47) (Figure 3). Life-threatening bleeding (Figure 4) and major vascular complications (Figure 4) occurred at a pooled estimate rate of 5.1% (95% CI: 3.3%-7.8%, I2=39.0) and 4.9% (95% CI: 3.5%-6.6%, I2=0), respectively. Major bleeding was seen in 10.5% (95% CI: 5.1%-20.4%, I2=77.56) of patients. The reported rates for a new permanent pacemaker implantation after TAVI ranged from 0% to 36.4%, with a pooled estimate rate of 13.5% (95% CI: 10.8%-16.9%, I2=58.5) (Figure 5). The occurrence of paravalvular AI of moderate or severe grade after TAVR was between 0% and 13.6%, with a pooled estimate rate of 3.2% (95% CI: 2.1%-5.0%, I2=39.26) (Figure 6). All the post-procedural complications are tabulated in Online Table 4.
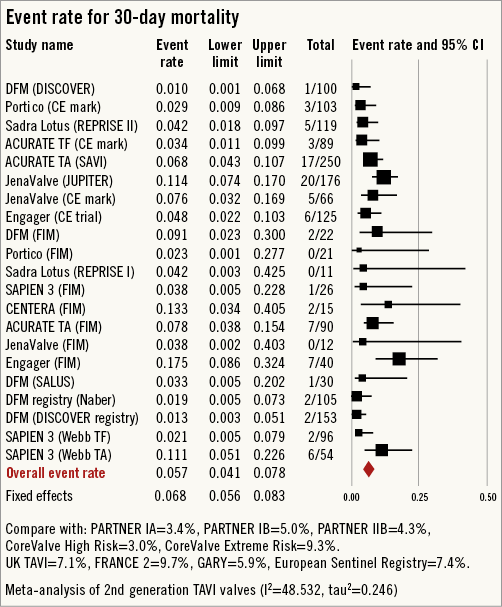
Figure 2. Forest plot showing the individual and pooled event rates for mortality after TAVI from the included studies.
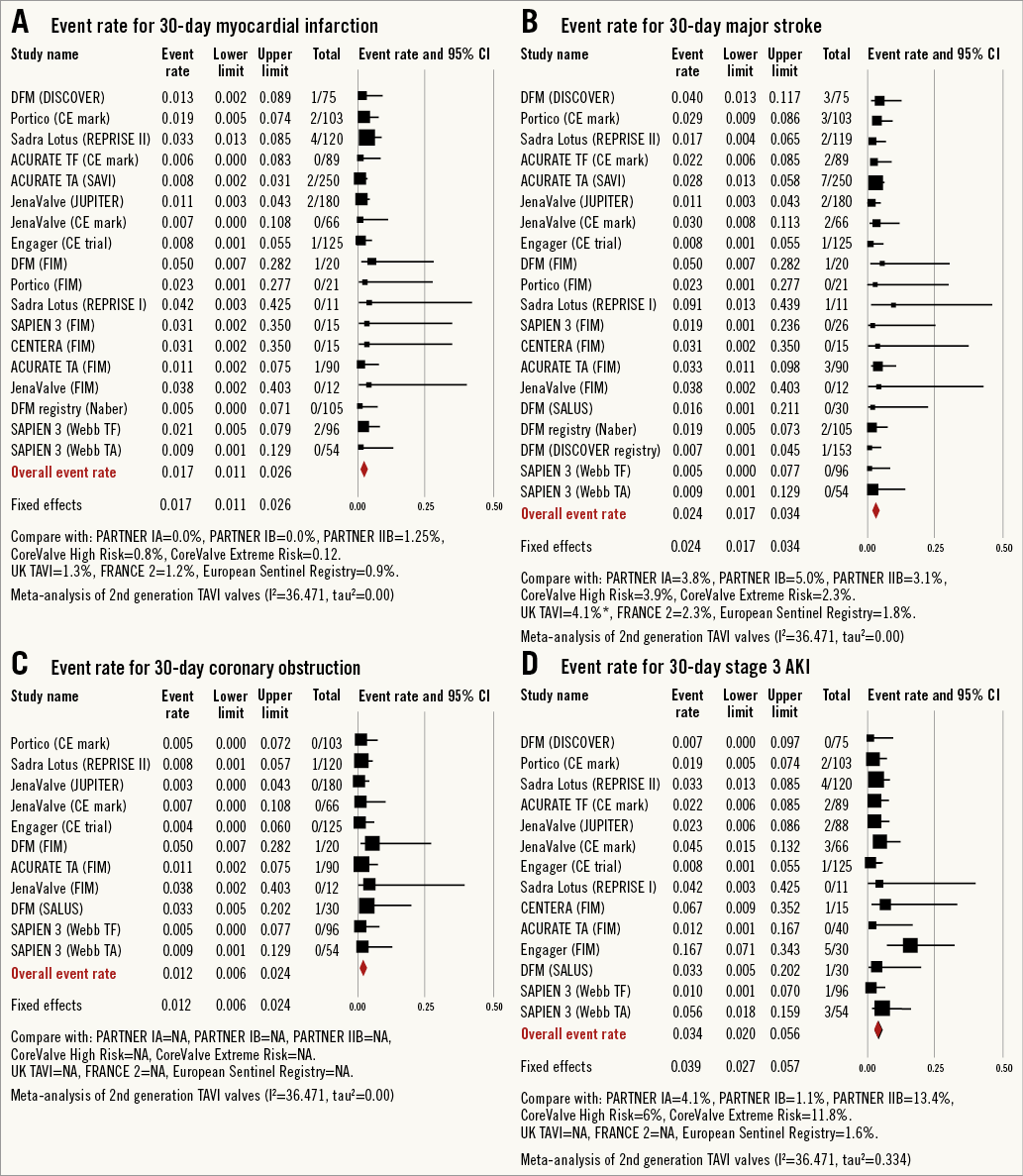
Figure 3. Forest plot showing the individual and pooled event rates after TAVI from the included studies for: A) myocardial infarction, B) major stroke, C) coronary obstruction (CO) and D) acute kidney injury (AKI) stage 3.
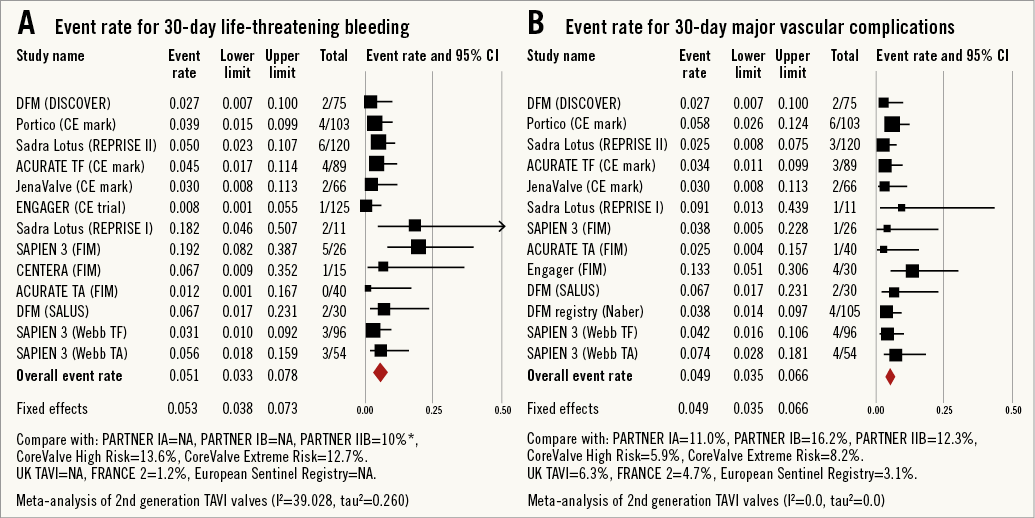
Figure 4. Forest plot showing the individual and pooled event rates after TAVI from the included studies for: A) life-threatening bleeding and B) major vascular complications.
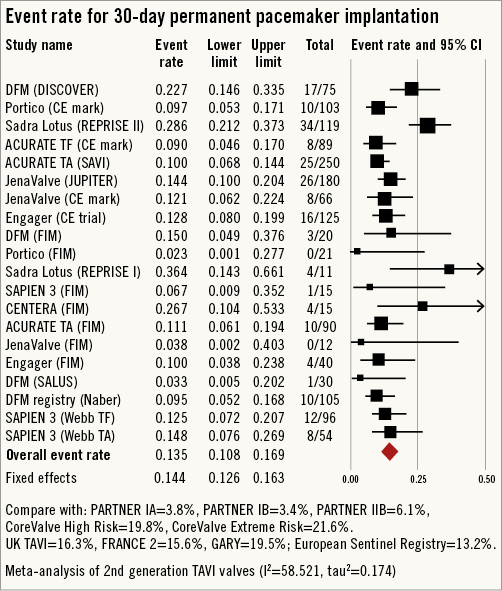
Figure 5. Forest plot showing the individual and pooled event rates for permanent pacemaker implantation after TAVI from the included studies.
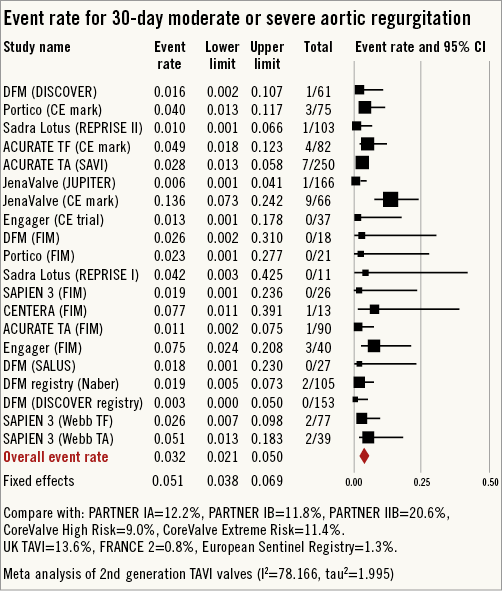
Figure 6. Forest plot showing the individual and pooled event rates for moderate or severe aortic regurgitation after TAVI from the included studies.
In the transfemoral group (Online Figure 1-Online Figure 9) the event rate for all-cause 30-day mortality was 3.4% (95% CI: 2.3%-4.9%, I2=0.0), for MI was 2.3% (95% CI: 1.4%-4.0%, I2=0.0), for AKI stage 3 was 2.6% (95% CI: 1.5%-4.5%, I2=0.0), and for life-threatening bleeding was 6.0% (95% CI: 3.7%-9.7%, I2=41.59). The major vascular complications event rate was 4.2% (95% CI: 2.9%-6.1%, I2=0.0), major stroke was seen in 2.5% (95% CI: 1.5%-3.9%, I2=0.0), permanent pacemaker implantation event rate was 14.6% (95% CI: 9.9%-21.0%, I2=69.17) and moderate or severe AI was 2.8% (95% CI: 1.8%-4.5%, I2=0.0).
In the transapical group (Online Figure 1-Online Figure 9) the event rate for all-cause 30-day mortality was 8.8% (95% CI: 6.6%-11.7%, I2=27.23), for MI was 1.0% (95% CI: 0.5%-2.1%, I2=0.0), for AKI stage 3 was 4.1% (95% CI: 1.7%-9.9%, I2=62.03), and for life-threatening bleeding was 2.9% (95% CI: 1.3%-6.5%, I2=11.23). The event rate for major vascular complications was 6.5% (95% CI: 3.1%-13.1%, I2=32.03), major stroke was seen in 2.3% (95% CI: 1.4%-3.8%, I2=0.0), permanent pacemaker implantation event rate was 12.1% (95% CI: 10%-14.5%, I2=0.0) and moderate or severe AI was 3.7% (95% CI: 1.6%-8.5%, I2=70.33).
Excluding FIM reports, the event rate for all-cause 30-day mortality was 4.7% (95% CI: 3.1%-6.9%, I2=58.62), for MI was 1.5% (95% CI: 0.9%-2.4%, I2=0.0), for AKI stage 3 was 2.8% (95% CI: 1.8%-4.3%, I2=0.0), and for life-threatening bleeding was 4.0% (95% CI: 2.7%-5.8%, I2=0.0). The event rate for major vascular complications was 4.3% (95% CI: 3.0%-6.1%, I2=0.0), major stroke was seen in 2.1% (95% CI: 1.5%-3.1%, I2=0.0), permanent pacemaker implantation event rate was 13.4% (95% CI: 10.2%-17.3%, I2=68.8) and moderate or severe AI was seen in 2.9% (95% CI: 1.6%-5.1%, I2=56.13).
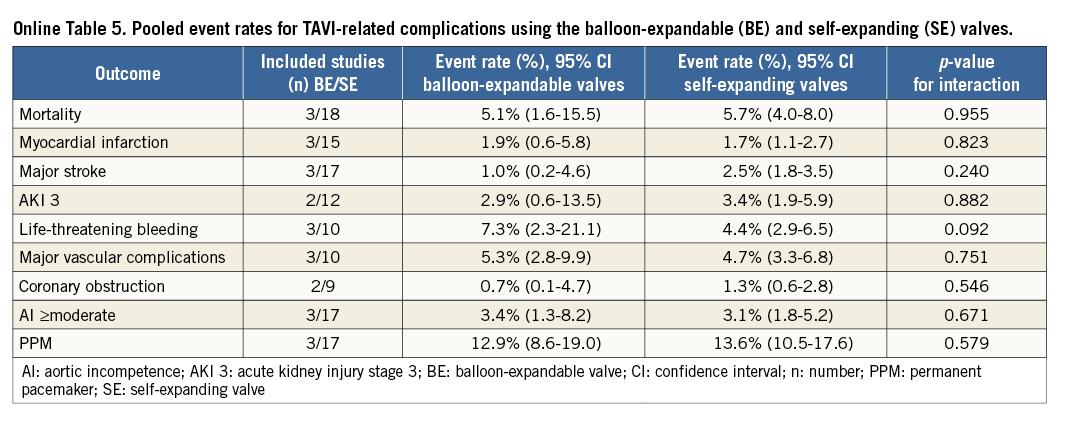
Pooled estimates of various outcomes according to studies reporting per VARC guidance and core lab utilisation, as well as balloon-expandable vs. self-expanding valves, are also provided in Online Figure 10, Online Figure 11 and Online Table 5, respectively.
Discussion
The current analysis, which includes 1,708 patients from 24 studies on eight unique second-generation THV (Figure 7), is the first pooled analysis reporting on the safety and efficacy of these new TAVI valves. The main results of the current analysis are as follows: 1) several new THV have entered the TAVI market with unique features and promising results, 2) these new THV have the potential to reduce several TAVI-related complications, 3) moderate to severe paravalvular AI after TAVI can be virtually eliminated with these new valves, 4) conduction abnormalities requiring permanent pacemaker implantation need to be addressed further in future designs, and 5) as a group, second-generation TAVI valves appear safe and effective in their initial experience; however, safety and efficacy at longer follow-up and of individual valves have yet to be determined.
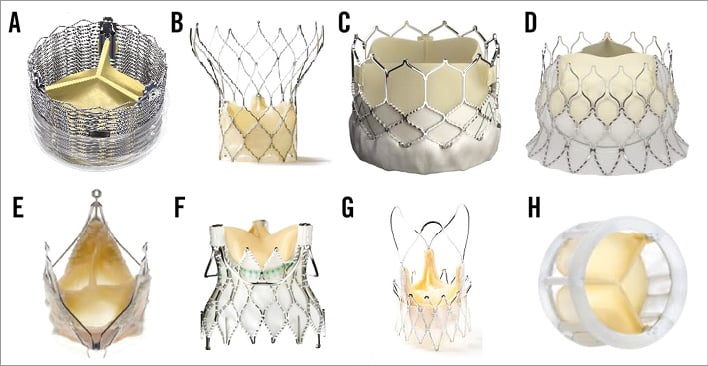
Figure 7. Second-generation transcatheter aortic valves. A) Sadra™ Lotus Medical valve (Boston Scientific SciMed Inc, Maple Grove, MN, USA); B) Portico® valve (St. Jude Medical); C) Edwards SAPIEN 3 valve (Edwards Lifesciences); D) Edwards CENTERA valve (Edwards Lifesciences); E) JenaValve (JenaValve Technology); F) Engager™ valve (Medtronic Inc.); G) Symetis ACURATE™ valve (Symetis SA); H) Direct Flow Medical® valve (Direct Flow Medical).
Some of the new THV have been engineered to improve the efficacy and safety of TAVI. Common features of these particular THV are retrievability and repositionability (resheathing) features which allow the operator to implant one valve, assess its size, position, haemodynamics and function in vivo, reposition it or remove it as needed, and implant a new valve when the valve is misplaced or is malfunctioning. Paravalvular AI is widely regarded as the “Achilles heel” of TAVI3. The ability to assess paravalvular leak early in the deployment process along with the ability to retrieve and reposition these newer valves has the potential to reduce paravalvular leak. Furthermore, each of these newer TAVI valves has unique features that improve positioning and sealing and which reduce paravalvular leak after TAVI.
In our pooled analysis of all reported studies using these aforementioned second-generation THV the risk of moderate or severe AI was 4.2% (95% CI: 2.0%-8.5%). The incidence of moderate or severe paravalvular AI after TAVI using first-generation THV was 11.7% (95% CI: 9.6%-14.1%) in a pooled analysis of 45 reports5. In the Placement of AoRTic TraNscathetER Valves (PARTNER) II trial39, moderate or severe AI was seen in 11.8% of patients implanted with the Edwards SAPIEN valve. In the Placement of AoRTic TraNscathetER Valves (PARTNER) II trial40 moderate or severe AI was seen in 24.2% of patients implanted with the Edwards SAPIEN XT valve and in 16.9% of patients implanted with the Edwards SAPIEN valve (p=0.12). Similarly, in the CHOICE trial41 moderate or severe AI was seen in 11.2% of patients (18.3% with the self-expanding and 4.1% with the balloon-expandable valve). In the CoreValve US pivotal trial42 paravalvular leak of moderate or severe intensity was observed in 11.5% of patients at 30 days. The inequality in incidence of post-TAVI AI among the aforementioned studies may be related in part to the variability in assessment. In the randomised PARTNER (Placement of Aortic Transcatheter Valve) trials and CoreValve US pivotal trial assessment of AI was performed by an independent echocardiographic core laboratory. In the CHOICE trial, assessment of AI was performed using an angiographic core lab. Assessment of post-TAVI AI with second-generation THV was performed by individual sites with or without core lab oversight. However, despite the differences in assessment, reduction in post-TAVI moderate or severe AI appears pronounced with second-generation THV.
Short-term survival was comparable to previously reported data from first-generation THV. Overall, the rate of other periprocedural complications including major vascular complications, major bleeding, myocardial infarction and AKI stage 2/3 were also low, despite the learning curve. There is a theoretical concern that repositioning and retrieving of the newer valves can lead to aortic injury and atheroembolism resulting in stroke. However, this was not borne out in our analysis. The risk of stroke in our pooled analysis was 2.4% (95% CI: 1.7%-3.4%). The risk of stroke with first-generation valves is in the order of 3.2% (95% CI: 2.1%-4.8%)3. The ultra-low profile of the second-generation delivery catheters is expected to reduce the risk of stroke from trafficking in the aortic arch, while the more controlled delivery of the newer devices is likely to reduce stroke during the deployment stage. Most second-generation TAVI valves do not require rapid pacing, which may be linked to functional cardiac arrest and possibly strokes in the watershed areas of the brain.
The incidence of new onset conduction system disease requiring permanent pacemaker implantation was 13.5% (95% CI: 10.8%-16.9%) in our pooled analysis of second-generation THV. In a weighted meta-analysis of first-generation TAVI valves the risk of pacemaker implantation was 13.9% (95% CI: 10.6%-18.9%)3. The risk of pacemaker implantation was more frequent with use of the Lotus valve (29.2%) and Edwards CENTERA valve (26.6%). Low rates of pacemaker implantation were reported by the other valves: Direct Flow valve (13.4%), JenaValve (13.1%), SAPIEN 3 (12.7%), Engager valve (12.1%), ACURATE TA/TF valve (10%) and Portico valve (8%). Similar findings of a low pacemaker implantation with the JenaValve were reported by Seiffert et al in their experience with second-generation THV43. However, unlike their experience, the Engager valve was not associated with higher rates of pacemaker implantation in our analysis. The higher rate of pacemaker implantation was attributed to the skirt of the Engager valve protruding into the left ventricular outflow tract. Whether the particular design increases conduction system disease needs further investigation. In line with the design of the Portico valve (lack of flared inflow) pacemaker implantation rates were the lowest (8.0%).
We acknowledge heterogeneity in our analysis. The I2 value for 30-day mortality, MI, major stroke, coronary obstruction, AKI, life-threatening bleeding, major vascular complications was less than 50%, suggesting low to moderate heterogeneity, but was >50% for the 30-day event rate for AI and PPM implantation (Figure 2-Figure 6). We investigated the cause of heterogeneity by conducting subgroup analyses and by exploring differences in the baseline clinical characteristics of the enrolled patients. However, substudy analysis (fixed vs. random effects) comparing the approach (TA vs. TF) did not show any significant difference. Subgroup analysis of core lab-reported AI was also similar to the overall pooled analysis (Online Figure 1-Online Figure 9). In addition, the pooled event rates for TAVI-related complications when comparing the balloon-expandable (BE) and self-expanding (SE) valves do not show significant variation. Clinical characteristics of the enrolled population in the individual studies were not dissimilar (Online Table 1A, Online Table 1B).
Limitations
There are several major limitations in this analysis. The second-generation TAVI valves are not homogeneous. Each second-generation THV has a unique design and concept which influences the safety and efficacy profile of the individual valves. We disregarded these differences in our analysis and pooled all valves assuming sameness. The experience with these devices was limited in number and also to a few experienced centres worldwide. Real-world outcomes may not be similar. Incomplete reporting was frequent. We also only pooled data that were available and clearly reported. This may have influenced our results for certain outcomes. Individual patient data analysis, though ideal, was not performed.
Conclusions
Several new second-generation TAVI valves have been designed to make TAVI simple and safe. The early results with these valves are promising with significant reduction in TAVI-related adverse outcomes. While none of them has been directly compared to first-generation TAVI devices, the results suggest improvement compared to first-generation valves. Nevertheless, safety and efficacy at longer follow-up and of individual valves have yet to be determined.
| Impact on daily practice The new THV which are entering the TAVI market have unique and useful features, appear to be safe, and have the potential to reduce several TAVI-related complications while maintaining valve efficacy. Moderate to severe paravalvular AI after TAVI can be virtually eliminated. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data