Abstract
Aims: Transcatheter aortic valve implantation (TAVI) is a novel treatment option for high surgical risk patients with severe symptomatic aortic valve (AV) stenosis. During TAVI, some patients may require emergent cardiac surgery (ECS). However, the incidence, reasons and outcomes of those needing ECS remain unknown.
Methods and results: We performed a search of the English medical literature using MEDLINE to identify all studies on TAVI and evaluate the incidence of ECS (i.e., within 24 hrs of TAVI) and outcomes for these patients. Forty-six studies comprising 9,251 patients undergoing transfemoral, transapical or trans-subclavian TAVI for native AV stenosis published between 01/2004 and 11/2011 were identified and included in this weighted meta-analysis. Overall, TAVI patients were old (mean=81.3±5.4 years) and had a high mean logistic EuroSCORE (24.4±5.9%). Few patients required ECS (n=102; 1.1±1.1%) and this was marginally higher among those undergoing transapical TAVI as compared to those undergoing transarterial TAVI (1.9±1.7% vs. 0.6±0.9%). Data on the reasons for ECS were available in 86% (88/102 patients) and 41% of these (36/88) were performed for embolisation/dislocation of the AV prosthesis, with aortic dissection (n=14), coronary obstruction (n=5), severe AV regurgitation (n=10), annular rupture (n=6), aortic injury (n=14), and myocardial injury including tamponade (n=12) constituting the rest. Mortality at 30 days was about 9-fold higher in patients who did need as compared with those patients who did not need ECS (67.1±37.9% vs. 7.5±4.0%).
Conclusions: Reported rates of ECS during TAVI were low with embolisation or dislocation of the prosthesis being the most common cause. ECS was associated with grave prognosis with two out of three patients dying by 30 days. Thus, refinement in TAVI technology should not only focus on miniaturisation and improving flexibility of the delivery systems and/or devices –which may have the potential for decreasing aortic dissection, annular rupture, and tamponade– but also incorporate modifications to prevent embolisation/dislocation of the valve.
Introduction
Transcatheter aortic valve implantation (TAVI) has become an established treatment for patients with symptomatic aortic valve disease deemed inoperable or at high risk for conventional surgical aortic valve replacement (AVR)1,2. Although being a less invasive catheter-based procedure, TAVI carries the potential risk of some complications that may ultimately require emergent cardiac surgery (ECS). Thus, TAVI procedures have been recommended to be performed with immediate cardiopulmonary bypass and surgical back-up available as standby for safety reasons3. However, the true incidence, reasons, and outcomes of patients requiring emergent cardiac surgery (ECS) in large number of those undergoing TAVI remain unknown as this information has not been consistently reported in published TAVI studies.
The aim of the current meta-analysis was to review all current published literature to study the reported incidence, reasons, and outcomes of ECS in patients undergoing TAVI.
Methods
DATA SOURCES AND STUDY SELECTION
Using the terms “transcatheter”, “transfemoral”, “transapical”, “percutaneous”, “aortic”, “valve” and “implantation”, a comprehensive search of the English-language medical literature was performed using the MEDLINE database to identify all studies on TAVI as previously described4. In brief, articles published between January 1st, 2004 and November 11th, 2011 were initially considered. Moreover, bibliographies of the retrieved articles were reviewed for additional potentially relevant studies. A multistage assessment was used to determine if articles would qualify for the analysis. Initially, only the abstracts were reviewed. Original publications including patients undergoing TAVI for native aortic valve disease either by the transfemoral, transaxillary/transsubclavian or transapical route were selected for data extraction. Case reports, reports on valve-in-valve TAVI for failed surgical bioprostheses as well as reviews were not included. Next, the full text versions of the selected articles were reviewed for data extraction. Multiple reports of previously listed patients by the same investigators were refined by including only the most recent data or data with most information on our study aims to avoid duplicate reporting. Studies were evaluated for the total number of patients provided in the respective publication. If available, the data from an individual study were separated into three groups according to the valve device and the access site used: group 1- Medtronic/CoreValve (MCV) ReValving system (Medtronic Inc., Minneapolis, MN, USA) transarterial (transfemoral and transaxillary/subclavian); group 2- Edwards SAPIEN (ES) (Edwards Lifesciences, Irvine, CA, USA) transarterial; group 3- Edwards SAPIEN transapical.
DEFINITIONS
ECS was defined as any cardiothoracic surgical intervention requiring cardiopulmonary bypass including the need for urgent aortic valve replacement, repair of myocardial injury or aortic injury or dissection, or pericardial drainage performed within 24 hours after TAVI. Surgical procedure(s) needed for vascular access site complications were not included in this definition.
STATISTICAL ANALYSIS
Rates of events were calculated as the number of events divided by the number of treated patients with available data. The approach to calculate individual rates for different studies and to combine these rates into a weighted average gives identical results if the weights are defined as the proportion of available patients provided in a specific study5. Results are presented as weighted mean±1 standard deviation.
Results
STUDY SELECTION
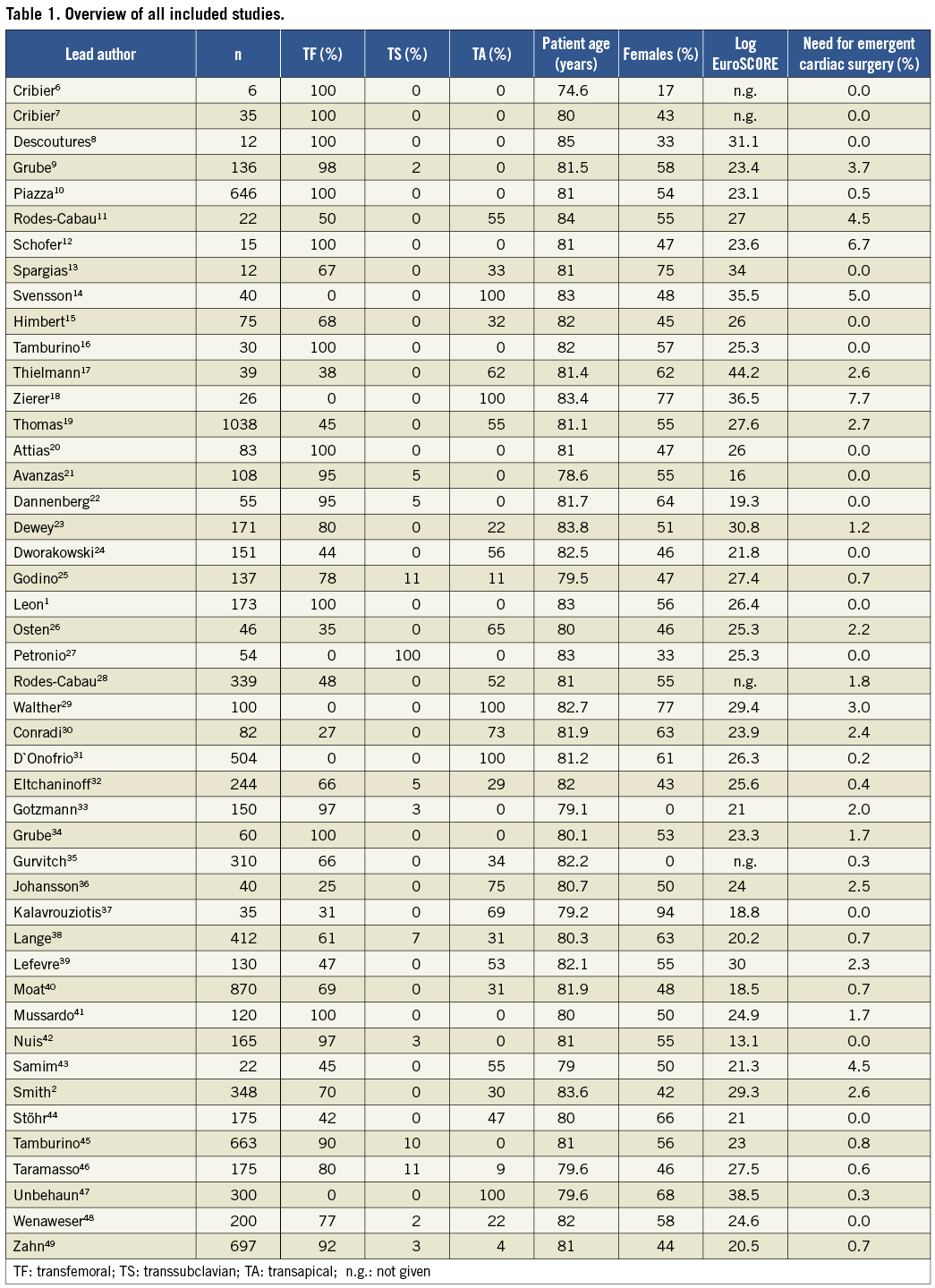
A comprehensive literature search identified 748 citations published within the predetermined time span of the search. After careful review, 46 studies comprising a total of 9,251 patients undergoing TAVI met the study criteria and were selected for the current analysis1,2,6-49 (Table 1).
STUDY POPULATION
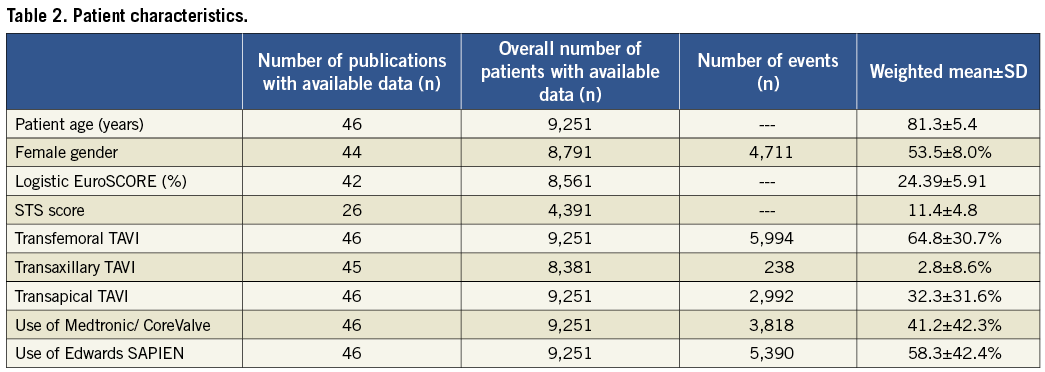
Characteristics of the overall patient population are detailed in Table 2. Patients were old with a mean age of 81.3±5.4 years and slightly over half were females (53.5±8.0%). The mean logistic EuroSCORE (24.39±5.91%) and the mean Society of Thoracic Surgeons (STS) score (11.4±4.8) were high. In 2/3 of patients, TAVI was performed via the transfemoral route and, in the remaining 1/3 of patients, a transapical approach was used. A minority of patients (2.8±8.4%) underwent TAVI using a transaxillary/subclavian access (Table 2).
EMERGENT CARDIAC SURGERY
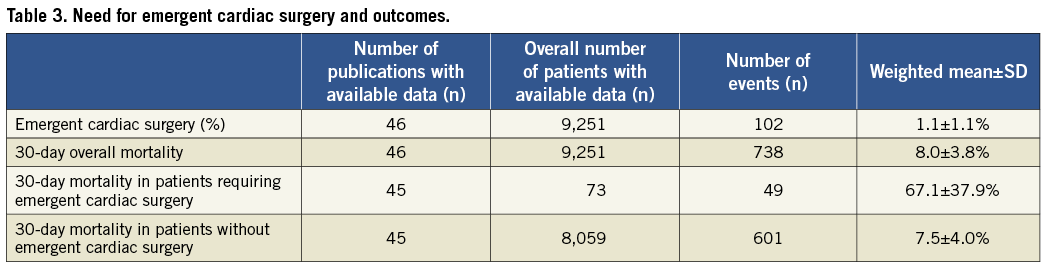
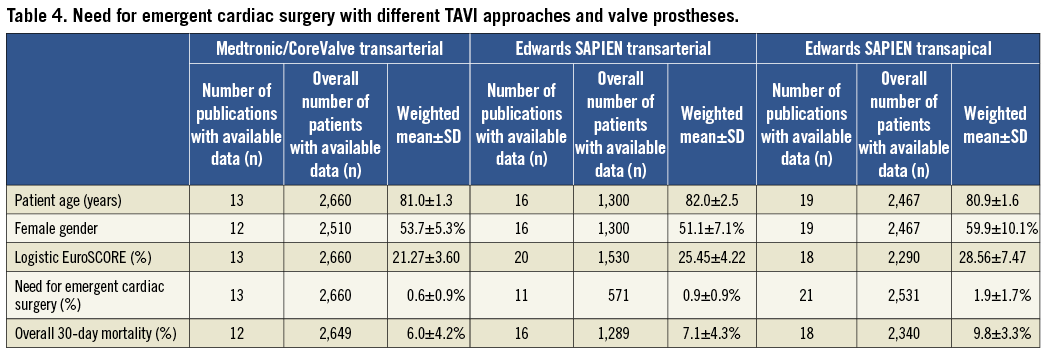
ECS was reported to be required in 102 out of the total of 9,215 patients (1.1±1.1%, Table 3). Lowest rates of ECS were observed for TAVI prostheses implanted transarterially (0.6±0.9%) and were comparable for the Medtronic/CoreValve (0.6±0.9%) and the Edwards SAPIEN (0.9±0.9%) devices (Table 4). Compared to transarterial TAVI, rates of ECS were twice as high in patients undergoing transapical TAVI (1.9±1.7%).
Information on the reasons for ECS was obtained from the papers or directly from the authors for 88 out of the total of 102 cases (86%, Figure 1). In 41% of these reported 88 cases, ECS was required for embolisation/dislocation of the implanted valve prosthesis (n=36) with aortic dissection/perforation (n=14), ventricular/atrial wall perforation resulting in bleeding or tamponade (n=12), severe AV regurgitation (n=10), aortic valve annulus rupture (n=6), and coronary obstruction (n=5) and others (n=5) comprising the rest (Figure 1).
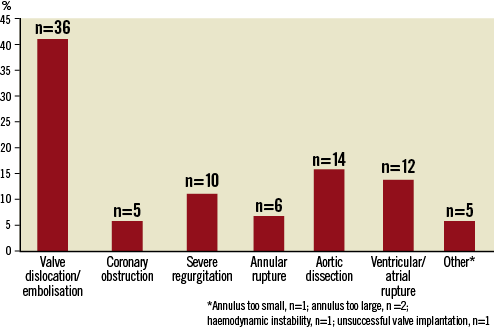
Figure 1. Overview of reasons for ECS.
Mortality rate of patients requiring ECS was 67.1±37.9% (transarterial 76.9±32.3% vs. transapical 58.6±43.4%) and was 9-fold higher compared with patients undergoing uneventful TAVI procedures (7.5±4.0%).
Discussion
The present meta-analysis of published TAVI literature comprising a total of 9,251 patients shows that the need for ECS during TAVI was low (1.1±1.1%). While ECS rates were low for transarterial as well as the transapical approach, this was more frequent with the latter technique. Among the studies that reported the reasons for ECS, the majority were performed for embolised/dislocated valve prostheses. The prognosis of patients requiring ECS was bleak with a 30-day mortality of 67%.
Our findings are consistent with more recent observational data from large registries. Similar to the current study, these data suggested low ECS rates between 0.1% and 0.4% and indicated that these rates were higher for the transapical than for the transarterial TAVI procedures50,51. However, these studies provided no insights into the reasons for ECS or into the outcomes of such patients.
Many relevant facts need to be kept in mind while interpreting these data and speculating about their implications. First, the real incidence of ECS may be somewhat higher than that reported in the above investigations and the studies in our meta-analysis. It is quite possible that ECS was not attempted in some of those who died of procedural complications because they did not have ECS for fear of surgical mortality in the otherwise high-risk cohort undergoing TAVI. Thus, some of the patients who died could possibly have been salvaged had ECS been attempted despite the high risk. If this was true, the current reported rate would be lower than the proportion of patients that would have been truly eligible for ECS. Nonetheless, it would still have been in single figures. Second, the higher rate of ECS with the transapical approach may be due to the fact that this strategy was more likely to be performed by cardiothoracic surgeons than by cardiologists and thus their threshold for immediate surgery during any TAVI complications may have been much lower. Alternatively, many studies have reported that patients undergoing the transapical approach have a higher risk profile (i.e., EuroSCORE and STS score) making them more susceptible to complications and thus accounting for the higher incidence of ECS3. Third, it is also possible that ECS was more common in patients undergoing the transapical procedure, as such an approach was traditionally performed either in a hybrid laboratory or in an operating room, thus providing an environment where ECS could be performed with much more ease. Fourth, the extremely high mortality reported with ECS should not be perceived as being prohibitive to offering surgery when deemed appropriate. Additionally, lack of a specific cause of death prohibits meaningful interpretation of the risks of ECS for the types of complications for which it is used. Thus, it is unlikely that ECS for embolisation or migration of the TAVI device would have as high a mortality as when it is utilised to treat aortic rupture or dissection.
The current incidence of ECS during the TAVI procedure should also be put in proper perspective with reference to the more traditional approaches for treating severe aortic stenosis, i.e., surgical aortic valve replacement. Most surgical literature reports only the incidence of reoperation for bleeding (the more common reason for reoperation after surgical AVR), but not other causes (minority) needing early reoperation, i.e., bypass graft failure, stuck/malfunctioning valve, aortic dissection, etc. Among 67,292 patients undergoing isolated AVR between 2002 and 2006 enrolled in the Society of Thoracic Surgeons National Cardiac Surgery Database (STS NCD), reoperation for bleeding was required in 5,369 (8%) of patients (33.9% ≥75 years of age)52. Similarly, among 159 patients undergoing isolated AVR at four large academic institutions in the US between 2002 and 2007 with STS risk score >10% (mean age 76.1+11.2 years), reoperation for bleeding was required in five (3.1%) patients53. No study has provided insight into the outcomes of reoperation for bleeding among patients undergoing isolated AVR. However, data from the STS in patients undergoing isolated CABG suggests a >4-fold increase in mortality among patients needing reoperation for bleeding54.
Our findings suggest the need for more studies to understand the reasons for ECS as well as to study why some patients with early procedural complications were not offered such an option. Similarly, data on complication-specific mortality should be collected in the future to have a better understanding of which patients are truly salvageable. This data would help provide guidance not only for identifying a subset of the already high-risk TAVI patients who are likely to benefit from ECS, but also for giving some insights into the futility of such procedures in others. Additionally, there is much debate currently among TAVI operators as to the need for cardiopulmonary bypass and/or surgical back-up. Current guidelines recommend that TAVI procedures have to be performed by the heart team consisting of a cardiologist and cardiothoracic surgeon with the immediate availability of a heart-lung machine during TAVI3. However, some sites without an on-site cardiothoracic surgical department continue to perform TAVI. Zahn et al49 report comparable mortality for patients undergoing TAVI at sites with (n=17) or without (n=5) on-site cardiac surgical capabilities (8.8% versus 3.8%, p=0.12), a result that the non-cardiac-surgical sites were able to achieve by collaborating with sites with cardiac surgical expertise in aortic valve surgery and with a cardiac surgical team visiting on-site during the procedure. Thus, while it should be strongly recommended that TAVI procedures be performed at cardiac surgical sites, non-cardiac-surgical sites that perform TAVI should be encouraged to take a heart team approach with collaboration and a visiting on-site cardiac surgical team (perhaps participating not only in the procedure but also in patient selection). These non-cardiac-surgical sites that perform TAVI should also be persuaded to participate in national TAVI registries and submit data on an on-going basis to these databases/registries so as to provide insight into the safety and effectiveness of such an approach at these non-surgical sites. Furthermore, a strong endorsement is made for hybrid operating rooms capable not only of TAVI, but for ECS (including general anaesthesia, cardiopulmonary bypass and surgical services) if required. Opponents of these recommendations indicate that investment in cardiopulmonary bypass or hybrid operating rooms is unwarranted and not cost-effective citing the current low need for acute conversion rates with their high mortality51. More data on the true incidence of complications benefiting from ECS as well as the cause-specific mortality may help guide or change these recommendations in the future to be more cost-effective, but without endangering patient safety. Finally, preventing the need for ECS through minimising some of the complications provides the best avenue for improving outcomes of TAVI patients. Given that the majority of ECS were performed for device embolisation or migration, technical improvements for preventing this during deployment, i.e., development of retrievable, re-positionable TAVI prostheses, as well as for facilitating easy retrieval of malpositioned or embolised devices may have significant potential for reducing ECS. Additionally, careful study of pre-procedural CT or MRI would not only allow appropriate positioning, but also allow for adequate post-procedural balloon dilation, particularly of the self-expanding valve to minimise malpositioning/embolisation while reducing the risk of aortic rupture and residual paravalvular regurgitation. Similarly, miniaturisation and improved flexibility of the delivery systems and/or devices may also help reduce ECS for complications such as aortic rupture or dissection.
Even though we evaluated the available information on more than 9,000 TAVI patients, given the lack of systematic reporting, the low rate of reported ECS and the lack of availability of patient level information, we suggest future efforts be directed at prospective and systematic collection of information on the incidence, cause and outcomes of patients undergoing ECS. This would not only allow more insight into the validity of our findings, but also help support or refute speculations regarding the implications of our study. While we made every effort to minimise overlapping of the same patients in different publications by the author(s) by including only data from their most recent publication, we cannot exclude some overlapping of patients, but the number of overlapping patients was probably relatively small.
Conclusions
Reported rates of ECS during TAVI were low with the most common cause being stated as embolisation/dislocation of the prosthesis. ECS was associated with grave prognosis with two thirds of these patients dying by 30 days. Thus, refinement in TAVI technology should not only focus on miniaturisation and improved flexibility of the delivery systems and/or devices –which may have the potential for decreasing aortic dissection, annular rupture, and tamponade– but also incorporate modifications to prevent embolisation/dislocation of the valve.
Conflict of interest statement
H. Eggebrecht and P. Kahlert are clinical proctors for Medtronic/CoreValve and Edwards Lifesciences and have received honoraria payments. The other authors have no conflict of interest to declare.

