
Abstract
Aims: The aim of this systematic review and meta-analysis was to investigate the predictors and outcome of acute kidney injury (AKI) after transcatheter aortic valve implantation (TAVI).
Methods and results: There were 35 articles recruiting 13,256 patients included in our study. Hypertension (odds ratio [OR] 1.92, 95% CI: 1.44 to 2.56), diabetes mellitus (OR 1.33, 95% CI: 1.20 to 1.47), peripheral artery disease (OR 1.28, 95% CI: 1.14 to 1.45) and a left ventricular ejection fraction <40% (OR 1.50, 95% CI: 1.19 to 1.88) were identified as significant independent predictors of AKI. In addition to the aforementioned comorbidities, procedure-related/post-TAVI factors such as transapical access (OR 1.68, 95% CI: 1.44 to 1.97), major bleeding (OR 1.82, 95% CI: 1.37 to 2.40) and transfusion (OR 1.30, 95% CI: 1.12 to 1.51) were also associated with a higher risk of AKI. Importantly, the risk of short-term all-cause death increased progressively with the aggravating severity of AKI (OR, 30 days: stage 1: 3.41; stage 2: 4.0; stage 3: 11.02; one year: stage 1: 1.95; stage 2: 2.82; stage 3: 7.34), as determined by a univariate analysis. After eliminating confounders, AKI remained linked to a higher risk for both short-term (30 days: HR 2.12, 95% CI: 1.59 to 2.83) and long-term (≥3 years: HR 1.37, 95% CI: 1.27 to 1.48) all-cause mortality.
Conclusions: The reason for the occurrence of AKI was multifactorial, including baseline characteristics, procedure-related and post-TAVI factors. It appeared that even stage 1 AKI exerted detrimental effects on survival within one year, and AKI was also independently linked to mortality beyond three years.
Introduction
There is increasing evidence to indicate that transcatheter aortic valve implantation (TAVI) may be an alternative to surgical aortic valve replacement (SAVR) in patients with either high-risk or inoperable aortic stenosis1. Acute kidney injury (AKI) after TAVI is a common complication, with an incidence ranging from 3% to 59.7%2,3. A previous systematic review has analysed the incidence and predictors of AKI; however, no pooled data regarding the predictors of AKI have been collected4. A recent meta-analysis demonstrated that AKI impacted on both 30-day and one-year all-cause and cardiac-cause mortality; however, the study did not eliminate confounders5. Moreover, the impact of AKI on long-term mortality and the impact of its magnitude on prognosis remain controversial6,7.
Therefore, we performed a systematic review and meta-analysis to investigate the predictors and outcomes of AKI especially based on its definition and severity.
Methods
STUDY IDENTIFICATION
A literature search of PubMed, EMBASE and CENTRAL was performed to identify articles reporting the predictors and outcomes of AKI in patients undergoing TAVI between January 2002 and April 2015 (Figure 1). Our search strategy was as follows: (TAVI OR TAVR OR transcatheter aortic valve OR transcatheter valve OR transcutaneous valve OR percutaneous valve) AND (AKI OR acute kidney injury OR renal failure OR contrast-induced nephropathy OR renal dysfunction OR CIN). Only studies published in English were included. We screened citations from titles and abstracts to full-text articles after discarding duplicate citations.
Figure 1. Flow diagram.
INCLUSION AND EXCLUSION CRITERIA
The inclusion criteria were as follows: (a) studies illustrating the risk factors and outcomes of AKI, reporting odds ratios (ORs), risk ratios (RRs) or hazard ratios (HRs), and (b) cohort studies including patients with AKI and patients without AKI. Studies were excluded for the following reasons: (a) the studies were abstracts, editorials, letters, reviews, comments or case reports, (b) the sample size was less than 50, (c) the studies utilised duplicate databases, or (d) the studies did not include human subjects. If ORs were reported by univariate and multivariate analysis simultaneously, only multivariate ORs were included.
DATA EXTRACTION AND QUALITY ASSESSMENT
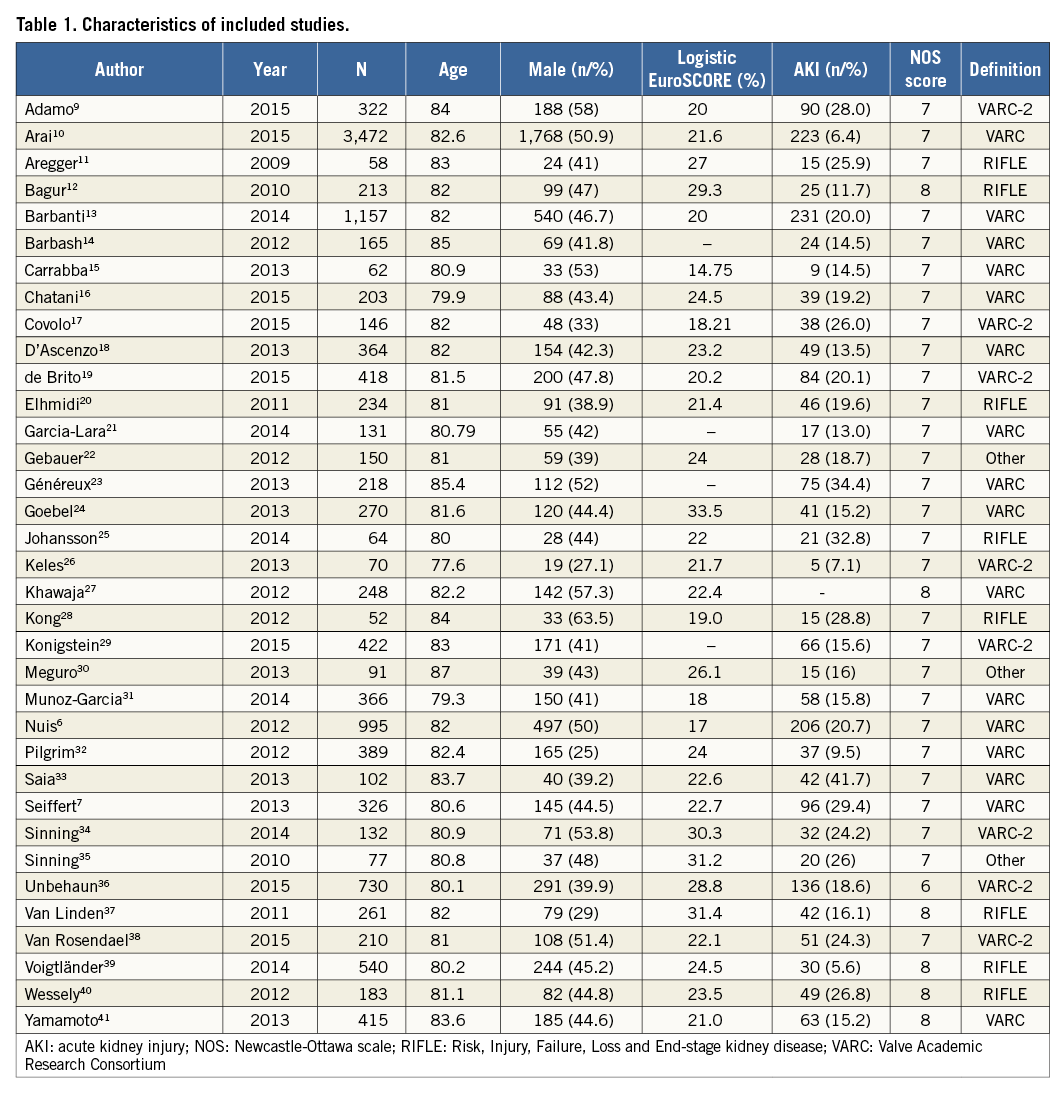
Two authors (Yan-biao Liao and Yang Meng) gathered the data independently, including the author, year, sample size, baseline characteristics and incidence of AKI (Table 1). Consensus was reached on all items. The quality of the included studies was assessed by the Newcastle-Ottawa scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm).
SUBGROUP ANALYSIS
We performed several subgroup analyses to investigate the effect of the varying severity of AKI on mortality.
PUBLICATION ANALYSIS
P-values on the Egger’s test greater than 0.05 and symmetry of the funnel plot determined the absence of publication bias. Publication bias analysis was performed when pooled studies were more than three. If significant publication bias was noted, Duval and Tweedie’s trim and fill method was used to acquire adjusted values.
DATA ANALYSIS
We pooled ORs using Comprehensive Meta-Analysis Software Version 2 (Biostat, Englewood, NJ, USA), whereas multivariate ORs and HRs were combined using RevMan Software Version 5 (The Cochrane Collaboration, London, United Kingdom). The inverse variance method was used to pool multivariate ORs and HRs. Only two or more ORs and HRs were pooled. If the I2 statistic was more than 50% and its p-value was less than 0.05, a random-effects model was used to obtain the combined effect estimates. Two-sided p-values less than 0.05 were considered statistically significant.
The present systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) group8.
Results
LITERATURE SEARCH AND STUDY SELECTION
The study selection process is presented in Figure 1. A total of 1,016 citations were retrieved after searching PubMed, EMBASE and CENTRAL database. There were 160 full-text articles assessed for eligibility after screening titles and abstracts. Thirty-five6,7,9-41 articles including 13,256 patients were ultimately included in the present systematic review and meta-analysis.
QUALITY ASSESSMENT
The quality of eligible cohort studies was assessed using the Newcastle-Ottawa scale, and the overall quality of these eligible studies was good (Table 1).
PREDICTORS OF AKI
UNIVARIATE ANALYSIS
Baseline characteristics
Previous myocardial infarction (MI)6,13,15,16,22,29,33-35,38,41 (OR 1.23, 95% CI: 1.02 to 1.49, I2=15.3, Egger’s=0.8) and chronic kidney disease (CKD)6,11-14,18,24,25,27,28,31,33-35,40,41 (OR 1.50, 95% CI: 1.02 to 1.49, I2=66.3, Egger’s=0.52) were identified as predictors of AKI, while coronary artery disease11,12,14,16,20,21,24,26,28,31,33-35,40, chronic obstructive pulmonary disease6,12-15,20,21,23-25,27-29,31,33-35,40,41 and pulmonary hypertension20,24,25,31,34,35,40,41 were not correlated with AKI (Figure 2).
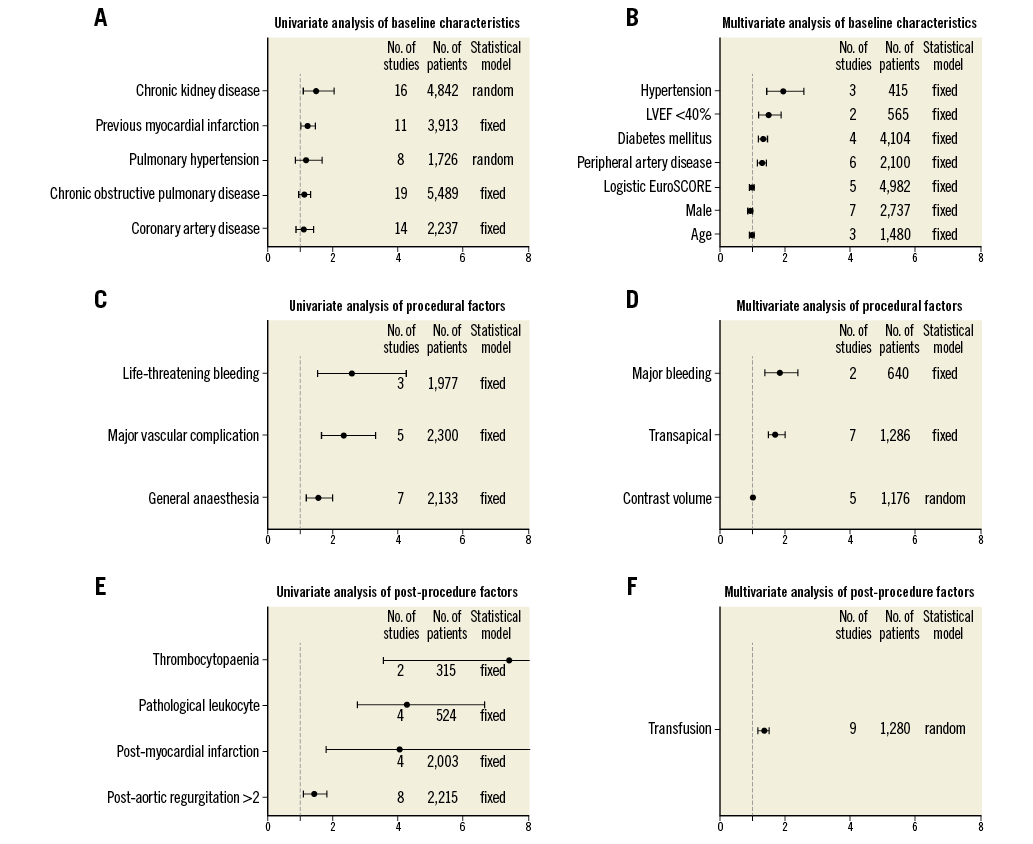
Figure 2. Predictors of acute kidney disease after transcatheter aortic valve implantation. LVEF: left ventricular ejection fraction
Procedure-related factors
General anaesthesia13,14,22,26,31,33,34 (OR 1.53, 95% CI: 1.18 to 1.98, I2=0, Egger’s=0.61), major vascular complications6,23,34,39,41 (OR 2.32, 95% CI: 1.63 to 3.30, I2=0, Egger’s=0.21) and life-threatening bleeding13,23,42 (OR 2.55, 95% CI: 1.52 to 4.27, I2=45.8) were associated with an increased risk for AKI (Figure 2).
Post-TAVI characteristics
Patients with a higher proportion of pathological leukocytes11,34,35,37 (OR 4.27, 95% CI: 2.73 to 6.67, I2=0, Egger’s=0.12) and more than grade 2 paravalvular leakage16,20,27,31,34,35,39,41 (OR 1.42, 95% CI: 1.1 to 1.82, I2=0, Egger’s=0.32) after TAVI appeared to be more susceptible to AKI. Thrombocytopaenia11,37 (OR 7.40, 95% CI: 3.56 to 15.39, I2=0) and MI12,13,23,41 (OR 4.05, 95% CI: 1.77 to 9.27, I2=11, Egger’s=0.21) after TAVI were associated with a higher risk for AKI (Figure 2).
MULTIVARIATE ANALYSIS
Baseline characteristics
Patients with a high logistic EuroSCORE6,10,16,33,38 (per increase: OR 1.01, 95% CI: 1.00 to 1.01, I2=23, Egger’s=0.20), hypertension12,22,28 (OR 1.92, 95% CI: 1.44 to 2.56, I2=5), diabetes mellitus (DM)10,20,22,27 (OR 1.33, 95% CI: 1.20 to 1.47, I2=15, Egger’s=0.68), peripheral artery disease (PAD)6,22,27,29,33,40 (OR 1.28, 95% CI: 1.14 to 1.45, I2=30, Egger’s=0.38) and left ventricular ejection fraction (LVEF) <40%22,41 (OR 1.50, 95% CI: 1.19 to 1.88, I2=5) were at increased risk for AKI, while age13,22,40 and male gender13,14,16,23,29,32,40 were not correlated with AKI (Figure 2).
Procedure-related and post-TAVI factors
Transapical (TA) access14,22,23,28,32,33,38 (OR 1.68, 95% CI: 1.44 to 1.97, I2=31, Egger’s=0.73), major bleeding23,29 (OR 1.82, 95% CI: 1.37 to 2.40, I2=0) and transfusion6,12,14,16,20,28,29,40,41 (OR 1.30, 95% CI: 1.12 to 1.51, I2=69, Egger’s=0.63) were identified as independent predictors of AKI (Figure 2).
OUTCOMES OF AKI
MULTIVARIATE ANALYSIS
We observed that patients with AKI experienced increased short-term, midterm and long-term all-cause mortality albeit that multivariate analysis showed a decreased trend over the follow-up (HR: 30 days6,13,23,35,40: 2.12, 95% CI: 1.59-2.83, I2=53, Egger’s=0.26; one year9,15,22,23,27,34: 1.7, 95% CI: 1.46-1.98, I2=0, Egger’s=0.80; two years6,16,30,31: 1.27, 95% CI: 1.06-1.53, I2=70, Egger’s=0.06 and ≥3 years13,17,19,29,36: 1.37, 95% CI: 1.27-1.48, I2=41, Egger’s=0.87). Furthermore, AKI showed an independent detrimental effect on 30-day to one-year interval survival13,19,23 (HR 1.36, 95% CI: 1.19-1.54, I2=0) (Figure 3).
UNIVARIATE ANALYSIS OF SEVERITY OF AKI AND MORTALITY
The pooled analysis of 106,7,9,13,16,23,24,33,34,36 studies demonstrated that the risk of all-cause death increased progressively with the aggravating severity of AKI (OR: 30 days: stage 16,24,33,34: 3.41, 95% CI: 1.89-6.13, I2=8.96, Egger’s=0.76; stage 224,33: 4.0, 95% CI: 0.43-36.97, I2=0; stage 324,33,36: 11.02, 95% CI: 5.68-21.38, I2=30.1; one year: stage 16,7,13,16,33,34: 1.95, 95% CI: 1.54-2.46, I2=49.6, Egger’s=0.34; stage 27,13,33: 2.82, 95% CI: 1.67-4.77, I2=0; stage 37,13,33: 7.34, 95% CI: 4.19-13.19, I2=0) (Figure 3).
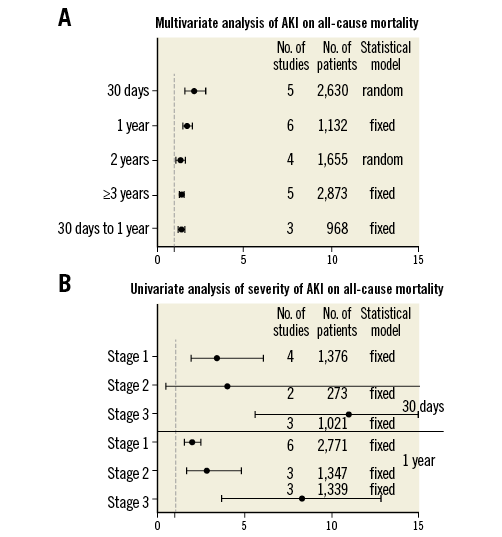
Figure 3. Impact of acute kidney injury on mortality.
Discussion
AKI is a common complication after SAVR, the incidence of which ranges from 4.1% to 57.7%1,43. TAVI has become an alternative to SAVR in patients with either high-risk or inoperable aortic stenosis1,2. In addition, a recent meta-analysis comparing the incidence of AKI between TAVI and SAVR demonstrated that TAVI had a lower incidence of AKI in studies with a propensity score and standard definition44. However, it is concerning that even stage 1 AKI impaired survival within one year in the present study, which was consistent with the findings of a previous study that included 995 patients6. Meanwhile, the impact of AKI on mortality increased with the aggravating severity of AKI. Moreover, AKI impaired not only short-term (30 days: twofold) but also long-term (beyond three years: 1.4-fold) all-cause mortality after adjusting for confounders. Similar results were also observed by Barbanti and co-workers13, who reported AKI impaired both early (<30 days) and late (>30 days) survival.
Regarding the predictors of AKI, we observed that the causes of AKI were multifactorial including baseline characteristics, procedure-related and post-TAVI factors. Patients with previous MI and lower LVEF were at increased risk of AKI, which may be related to “cardiorenal” syndrome, which impairs kidney perfusion and function14. Patients with stage 2 post-aortic regurgitation (AR) or worse and post-MI, which impacted on their haemodynamic stability, exhibited impaired diastolic renal blood flow and diminished renal function41. Therefore, careful management of cardio-circulatory homeostasis and adequate renal perfusion via the combination of blood pressure and the renal resistance index (RRI <0.85) is vital for preventing the occurrence of AKI34.
It appeared that PAD was an independent risk factor for AKI in the present meta-analysis, which was consistent with the findings of a previous study27. This result may account for the higher burden of atherosclerotic plaques, particularly in the ascending aorta and aortic arch, which may be disrupted by TAVI performance, resulting in the occlusion of small renal arteries38. Moreover, atherosclerotic renal disease may also play a role in the deterioration of kidney function33. Hypertension and DM impair renal autoregulation; therefore, elderly patients with hypertension and DM may be susceptible to suffer AKI after TAVI45. This phenomenon was also observed in patients undergoing cardiothoracic surgery and percutaneous coronary intervention21. Previous studies have demonstrated that CKD is associated with an increased risk of AKI after cardiac surgery, which is consistent with observations made in the setting of TAVI46. Patients with CKD are vulnerable to nephrotoxic drugs, renal hypoperfusion, and inflammatory mediators during TAVI; therefore, they are also at increased risk for AKI.
The present study established that major bleeding and transfusion were predictors of AKI. The transfusion of preserved red blood cells (RBC) may impair renal function due to structural and functional changes in the preserved RBC, with the accumulation of pro-inflammatory mediators47. Moreover, Nuis et al6 demonstrated that the impact of RBC transfusion on AKI and long-term mortality intensified with increasing numbers of RBC transfusions. Meanwhile, post-procedure anaemia and haemoglobin decreases were associated with a 1.8-fold higher risk of AKI10. Therefore, efforts should be made to reduce vascular complications, bleeding and transfusions6.
TA access is a more invasive approach and may impair dynamic stability to a greater extent and induce systemic inflammatory response syndrome (SIRS) at a higher rate compared with transfemoral (TF) access28. TA access always requires general anaesthesia, which is also a risk factor for TAVI13. In addition, TA access is chosen in cases in which peripheral artery access is not safe for TAVI because of the higher arteriosclerotic burden. Therefore, TA access was associated with a twofold higher risk of AKI in the present study, a finding consistent with previous studies28.
A relationship between leukocyte count and AKI was observed, which was also noted in a previous study47. Sinning et al47 observed that only major vascular complications and the number of ventricular pacing runs were independent predictors of SIRS. However, Retting et al48 identified no risk factors for SIRS. Both studies demonstrated that the multi-organ injury caused by reduced cardiac output was a major pathologic mechanism of SIRS, which increased the risk of AKI. Therefore, every effort should be made to reduce the period of hypotension caused by rapid pacing, balloon aortic valvuloplasty and valve deployment. Moreover, statin initiation before cardiovascular surgery may reduce the incidence of AKI; thus, future studies should also investigate whether statin initiation before TAVI reduces the occurrence of AKI49.
Finally, we observed that the amount of contrast media was not an independent predictor of AKI, which was consistent with the findings of previous studies23,40. However, some other studies observed a significant correlation between contrast medium and AKI16,37. This difference may be explained by the fact that only when the amount of contrast medium exceeded a threshold value (contrast medium×serum creatinine/body weight >2.7 or contrast medium/creatinine clearance >3.7) was this variable associated with an increased risk of AKI41. However, it is noteworthy that contrast medium may accelerate the deterioration of kidney function in patients with other predictive factors; therefore, hydration before and after TAVI may reduce the risk of AKI22.
Limitations
There were several limitations in the present study. First, several combined results were obtained by pooling small numbers of studies which were not so convincing. Second, some of the predictors and outcomes of AKI were pooled without excluding potential confounders. Finally, although efforts were made to eliminate overlapping data by author, centre and patient recruitment time, duplicate data may have been included in our study.
Conclusions
It appeared that even stage 1 AKI exerted detrimental effects on survival within one year, and overall AKI was also independently linked to mortality beyond three years. The pathogenesis of AKI was multifactorial, which included patients’ baseline characteristics, procedure-related and post-TAVI factors such as hypertension, PAD, DM, TA access, major bleeding and transfusions.
| Impact on daily practice Acute kidney injury has a detrimental effect on survival. Thus, efforts should be made to reduce the incidence of major bleeding and transfusion, which are predictors of AKI, especially in patients with risk factors of AKI after TAVI such as hypertension, diabetes mellitus, peripheral artery disease and using transapical access. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

