Abstract
Aims: The aim of this study was to identify the incidence and risk factors for acute kidney injury (AKI) after TAVI, a potentially serious complication of transcatheter aortic valve implantation (TAVI) that has been redefined by the Valve Academic Research Consortium (VARC).
Methods and results: We performed a retrospective analysis of 248 patients undergoing TAVI. AKI was defined as a VARC-modified Risk, Injury, Failure, Loss, and End-stage (RIFLE) kidney disease score ≥2. Eighty-nine patients suffered AKI (35.9%) and demonstrated increased mortality at 30 days (13.5% vs. 3.8%) and one year (31.5% vs. 15.0%) (p<0.001). Multivariate regression analysis identified diabetes mellitus (p<0.001), peripheral vascular disease (p=0.007), chronic kidney disease stage (p=0.010) as independently associated risk factors for AKI.
Conclusions: More than one third of patients sustain AKI after TAVI using the Edwards bioprosthesis, as defined by the VARC-modified RIFLE score. AKI increased the mortality at both 30 days and at one year. A history of diabetes mellitus, peripheral vascular disease and higher chronic kidney disease stage had the strongest independent associations with post-TAVI AKI.
Introduction
The last few years have witnessed a paradigm shift in the treatment of severe aortic stenosis in high-risk patients. Previously these patients often did not receive the existing gold standard of surgical aortic valve replacement (sAVR)1 due to prohibitive peri-operative risk, caused by multiple comorbidities and advanced age2. This cohort has now benefitted from the introduction of transcatheter aortic valve implantation (TAVI). This has been shown to have comparable results to sAVR in high-risk patients3, clear mortality benefits when compared to optimal medical treatment4 and to be cost-effective5. The safety and efficacy of this therapy is also manifest in observational data from large, international registries6-8. With a growing evidence base the uptake has been rapid and enthusiastic with co-operation between the interventional cardiology and cardiac surgical communities9.
Acute kidney injury (AKI) after cardiac surgery is associated with increased mortality and necessity for renal replacement therapy (RRT) even more so10,11. Similarly AKI after TAVI is not infrequent, affecting 11.7-27.8% of patients in previous series12-15. The definition of AKI in these series was according to the “risk, injury, failure, loss, end-stage (RIFLE)” criteria16. The recent consensus from the Valve Academic Research Consortium (VARC) proposed a modified RIFLE criteria for the reporting of AKI in TAVI patients that removed some of the ambiguity associated with the original criteria with regard to the loss and end-stage definitions and variation in renal replacement therapy provisions, recognised smaller deteriorations in function in the less severe stages, specified a 72-hour rather than a 48-hour period to ensure association with the TAVI procedure itself and formally eliminated urine output measurement from the definition17. Our aim in this study was to investigate the incidence and effects of AKI as newly defined by VARC–and to identify preprocedural risk factors that may allow us to identify those most at risk.
Methods
Between March 2008 and November 2011 250 patients were enrolled into the TAVI programme at St. Thomas’ Hospital, London. As part of this, data were collected prospectively onto a TAVI-specific database. One patient was excluded due to existing chronic kidney disease (CKD) requiring haemodialysis prior to TAVI and one patient underwent a trans-subclavian TAVI using a CoreValve device (Medtronic, Minneapolis, MN, USA). We performed a retrospective analysis on the remaining 248 consecutive patients. Parameters included were: age, gender, logistic EuroSCORE, left ventricular ejection fraction (LVEF), peak transaortic gradient (mmHg), dyspnoea according to New York Heart Association (NYHA) score, previous cerebrovascular disease, peripheral vascular disease, hypertension, diabetes mellitus, chronic pulmonary disease, previous percutaneous coronary intervention (PCI), previous coronary artery bypass grafting (CABG), PCI in preparation for TAVI, contrast media used for TAVI and contrast dose.
TAVI PROCEDURE
Patients referred for consideration of TAVI underwent a systematic assessment that included transthoracic echocardiography (TTE), coronary angiography, aortography and ilio-femoral angiography, computed tomography (CT) scan of the aorta, pulmonary function testing, carotid artery ultrasonography and independent assessments by both a cardiac surgeon and an interventional cardiologist. Each case was then considered at a multidisciplinary meeting (MDT) that included interventional cardiologists, imaging-specialist cardiologists and cardiac surgeons. Patients were accepted for TAVI according to accepted guidelines if either they were found to be at excessive peri-operative risk for sAVR (logistic EuroSCORE ≥20), they were turned down for sAVR by two cardiac surgeons or if sAVR was contraindicated due to technical considerations18.
TAVI was performed under general anaesthesia as previously described via the transfemoral, transapical, trans-subclavian or transaortic approaches19-21. Both two- and three-dimensional transoesophageal echocardiography (TEE) were utilised periprocedurally. All patients received the balloon-expandable Edwards SAPIEN bioprosthesis, available in 23 millimetre (mm), 26 mm and 29 mm diameter sizes (Edwards Lifesciences, Inc., Irvine, CA, USA).
DEFINITION OF AKI
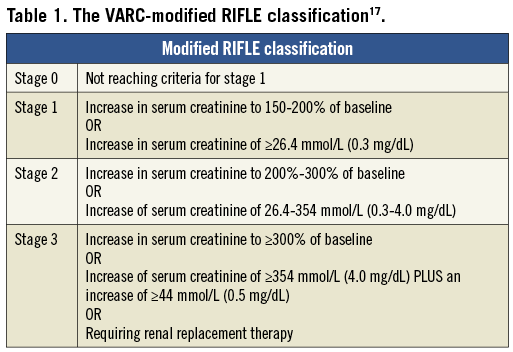
All patients had systematic measurement of serum creatinine prior to TAVI and at 48 hours (h) and 72 h post procedure. Each patient was then coded for the maximum VARC-modified RIFLE score (Table 1) and AKI was defined as stage 2 or 3 as stipulated17. If patients required renal replacement therapy (RRT) they were classed as stage 3 as stipulated by VARC. The decision to commence RRT and indications for such intervention was made by the intensivists or nephrologists responsible for the patients’ clinical care.
STATISTICAL ANALYSIS
Statistical analysis was performed using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as mean±standard deviation (SD) or median (interquartile range) and were compared using the Student t-test or a non-parametric test where a Shapiro-Wilk test suggested non-normal distributions. Categorical variables are expressed as percentages and were compared using χ2-tests. Univariate logistic regression was performed and any promising variables (i.e., p<0.10) were used to create a multivariate logistic regression model using a forward elimination method and an inclusion criteria of p<0.05. Continuous variables with a non-normal distribution were transformed using the natural logarithm for this analysis. Survival analysis was performed using the Kaplan-Meier method and a log rank test performed for comparison. Mortality data were obtained from the Office of National Statistics (ONS) and procedural complications were followed up in clinic or by telephone. No patients were lost to follow-up in these respects.
Results
The baseline characteristics of the study population are summarised in Table 2. Of 248 patients who underwent TAVI using the Edwards bioprosthesis 89 (35.9%) patients suffered an acute kidney injury as defined by the score of ≥2 on the VARC-modified RIFLE score. The individual frequencies of the VARC-modified RIFLE score are illustrated in Figure 1. There were no significant demographic differences between patients in the two groups, including age (mean age AKI patients was 83.3±6.9 years versus 81.7±9.8 years, p=0.346), male gender (61.8% vs. 54.7%, p=0.280) and mean logistic EuroSCORE (25.3±16.1% vs. 20.7±10.9%, p=0.128). There was a significant difference in the access used in patients with and without AKI with fewer patients receiving transfemoral TAVI (TF-TAVI) developing AKI compared to those who had an alternative access route, either transapical (TA-TAVI) or transaortic (TAo-TAVI) (32.6% vs. 44.9% vs. 22.5%, p=0.014). Patients who developed AKI also had lower transaortic gradients, both peak (65.7±23.9 vs. 74.5±25.1 mmHg, p=0.009) and mean (40.1±16.2 vs. 46.6±17.4 mmHg, p=0.006). There was no significant difference in the rate of post-procedural stroke between the AKI and no AKI groups (6[3.9%] vs. 6[7.2%], p=0.296). There was no association found between the presence of significant post-procedural AR (≥ grade 2 defined echocardiographically or angiographically) and incidence of AKI (36.0% vs. 36.2%, p=0.975).
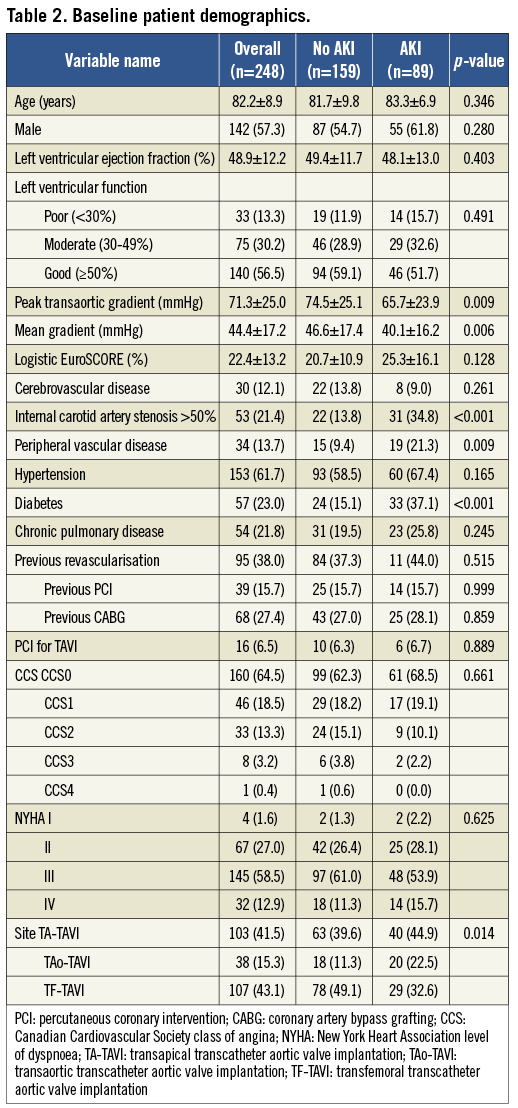
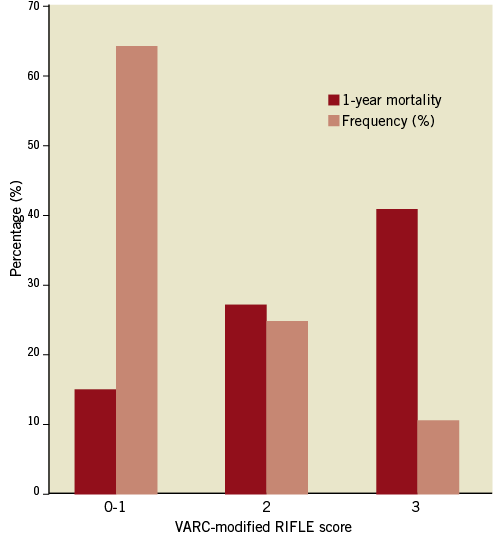
Figure 1. Frequency of VARC-modified RIFLE score and associated 1-year mortality.
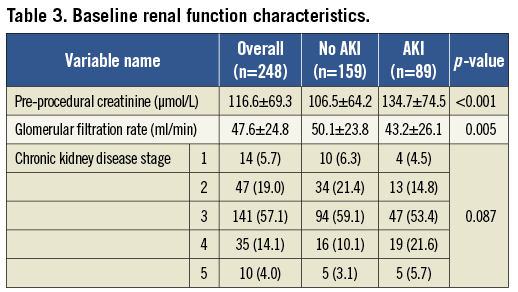
The overall mean pre-procedural creatinine was 116.6±69.3 µmol/L with an overall peak creatinine of 148.8±94.4 mmol/L (p<0.001). Patients with AKI had greater mean pre-procedural (134.7±74.5 vs. 106.5±64.2 mmol/L (p<0.001), 48 h (206.8±89.1 vs. 98.0±40.0 mmol/L (p<0.001)) and 72 h creatinine concentrations (205.9±88.3 vs. 99.3±39.6 mmol/L (p<0.001)). 70 (28.1%) patients saw an improvement in serum creatinine from baseline to 72 h. Twenty-five patients required renal replacement therapy (10.0%). The renal characteristics are summarised in Table 3. The mean dose of contrast used to perform TAVI was 107.3±48.3 mls. There was no significant difference in contrast dose between patients who subsequently developed AKI and those who did not (110.35±47.5 mls vs. 101.1±49.1 mls, p=0.068). Five patients underwent coronary angiography ≤5 days prior to TAVI and of these two suffered AKI: the mean contrast dose of these patients was higher (205±7.1 mls vs. 146.7±20.8 mls, p=0.035).
AKI AND MORTALITY
A higher VARC-modified RIFLE score was associated with increased mortality (p<0.001). Kaplan-Meier analysis (Figure 2) according to incidence of RIFLE score ≥2 (i.e., AKI) demonstrated significantly increased mortality at 30 days (13.5% vs. 3.8%), one year (31.5% vs. 15.0%) and overall (40.4% vs. 19.5%; log rank p<0.001) at a median follow-up of 379 days (interquartile range 113-729 days).
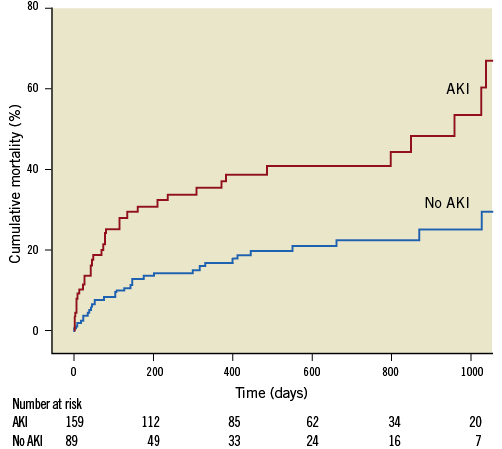
Figure 2. A Kaplan-Meier curve showing increased mortality associated with AKI as defined by a VARC-modified RIFLE score of ≥2 (log rank <0.001).
FACTORS ASSOCIATED WITH AKI (TABLE 4)
Univariate analysis of pre-procedural risk factors, as well as procedural factors such as contrast dose and severe AR, identified the logistic EuroSCORE (odds ratio 1.03, 95% confidence interval 1.01-1.05, p=0.012), pre-procedural stage of chronic kidney disease (OR 1.47, 95% CI 1.07-2.03, p=0.019), peak transaortic gradient (OR 0.99, 95% CI 0.97-1.00, p=0.009), peripheral vascular disease (PVD) (OR 2.61 95% CI 1.25-5.43, p=0.011), and diabetes mellitus (DM) (OR 3.32, 95% CI 1.80-6.11, p<0.001) as variables of interest.
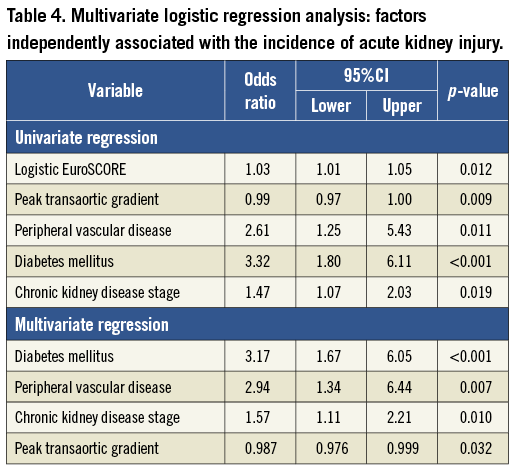
Subsequent multivariate logistic regression analysis revealed that the variable with the strongest independent association with risk of AKI was DM (OR 3.17, 95% CI 1.67-6.05, p<0.001), followed by peripheral vascular disease (OR 2.54, 95% CI 1.34-6.44, p=0.007) and the pre-procedural stage of chronic kidney disease (OR 1.57, 95% CI 1.11-2.21, p=0.010). Higher peak transaortic gradient was associated with a slight reduction in risk (OR 0.99, 95% CI 0.98-1.00, p=0.032).
FACTORS RELATED TO MORTALITY WITHIN ONE YEAR OF TAVI
A multivariate logisitic regression model was similarly created for mortality at one year. The factors showing independent association were post-procedural stroke (OR 62.76 95% CI 7.50-525.12, p<0.001), AKI (OR 2.47 95% CI 1.24-4.92, p=0.010) and a small association with higher logistic EuroSCORE (OR 1.03 95% CI 1.00-1.05, p=0.022).
Discussion
The incidence of VARC-defined AKI in the study TAVI population was 35.9% and 10.0% required renal replacement therapy during the index hospital stay. The AKI group had higher mortality with a greater than 3.5-fold increase in mortality at 30 days and 2-fold at one year compared to the group without AKI. AKI was also found to have an independent association with risk of death at one year with increased risk of almost 2.5-fold. Previous studies have used the original RIFLE definitions of acute kidney injury and required an increase in serum creatinine of ≥150% or >25% loss of glomerular filtration rate (GFR)16 rather than the more sensitive minimum criteria for definition of AKI of a rise of >26.4 mmol/L in the VARC-modified RIFLE classification (or a >200% increase in creatinine)17. This may explain the lower rates of between 12% and 28% seen in these studies12,13,15 rather than our cohort representing a higher risk group. The definition of AKI as a VARC-modified RIFLE score of ≥2 would seem to broaden the scope of AKI diagnosis. However, similar to our results, the AKI group in each study had significantly increased mortality. The risk of requiring RRT in patients undergoing sAVR is less than 5%22. Our rate of RRT use was higher at 10%, likely reflective of the higher risk nature of the TAVI cohort. Other TAVI series in patients receiving the Edwards bioprosthesis have described lower rates of RRT4,6. The decision to initiate RRT in our patients was made by the intensivists and nephrologists at our hospital with a lower threshold mindful of the deleterious impact of AKI in sAVR and TAVI.
AKI in the post-cardiac surgery period is typically the result of renal hypoperfusion and reduced oxygen delivery23. This leads to degradation of adenosine triphosphate (ATP) and loss of mitochondria, causing further loss of ATP24. Decreased ATP levels cause a variety of detrimental effects including renal endothelial and epithelial cell dysfunction and release of calcium ions25. Renal hypo-perfusion also promotes adenosine availability, leading to local vasoconstriction and reduced blood flow, and xanthine oxidase activity, forming the reactive oxygen species thought to mediate reperfusion cell injury26. In patients undergoing cardiac surgery this process is thought to be exacerbated by recent administration of nephrotoxic intravenous contrast27,28. Whilst TAVI patients do not suffer the insult of cardiopulmonary bypass, there is a period of hypotension during rapid ventricular pacing, routinely used during preparatory balloon aortic valvuloplasty and the TAVI itself to reduce cardiac output and allow stable device deployment. This may represent a risk period for AKI due to impairment of renal perfusion as suggested by Mehran et al29.
AKI after percutaneous coronary intervention is often attributed to contrast nephropathy29-31 and this is also possibly a significant contributor to the AKI mechanism in TAVI. However, we found no association between the incidence of AKI and increased contrast dose as a continuous variable nor when stratified by increments of 100 mls (p=0.245) –as proposed in the risk-scoring system proposed by Mehran et al for post-PCI contrast nephropathy29. It may be that our study is not powered sufficiently to identify a more subtle association. Their study also details increased risk of nephropathy with hypotension and diabetes mellitus. The latter was found in our study to be independently associated with risk of AKI, with an increased risk of more than 2.5-fold. Higher rates of AKI were seen in non-TF-TAVI patients despite these patients receiving larger doses of contrast than either TA-TAVI or TAo-TAVI (123.9±45.0 mls vs. 97.9±50.2 mls (p<0.001) and 123.9±45.0 mls vs. 83.8±33.7 mls (p<0.001), respectively) suggesting other underlying factors increasing risk between these access groups. The renovascular disease associated with the microvascular and macrovascular complications of diabetes may make the renal tissue more vulnerable to the effects of hypoperfusion, as well as impairing the intrinsic repair mechanisms. Similar renal processes can be expected in those suffering from PVD, as there is likely to be associated renovascular disease.
Previous studies have suggested that cardiac surgery within 24 hours of contrast use in coronary angiography increased the risk of AKI, though this may represent the relatively poor state of patients requiring such urgent treatment28,32. A larger study by Del Duca et al suggested that angiography within five days was independently associated with AKI after cardiac surgery33. More recently a yet larger study has demonstrated no effect upon incidence of AKI at the same cut-off34. Of five patients in our study who underwent TAVI at ≤5 days after angiography only two developed AKI, but there was a significantly higher total contrast dose. We would suggest that TAVI soon after angiography should be avoided and, if performed, care must be taken to minimise contrast load.
Similar rates of renal failure have been reported with CoreValve use compared to use of the Edwards system35,36. Wenaweser et al did find higher rates of stage 3 renal failure in CoreValve use compared to TF-TAVI using the Edwards prosthesis –but the highest rate was observed in the Edwards TA-TAVI cohort37. The CoreValve system does not typically require rapid ventricular pacing (and its associated hypotension) during valve deployment - though both systems require its use during preparatory balloon aortic valvuloplasty.
It is likely that the pathophysiology of AKI is multifactorial, involving predisposition of some patients to injury (through DM, PVD etc.), contrast nephropathy, embolic phenomena and hypoperfusive injury.
Greater pre-operative serum creatinine concentration only suggested a weak association with AKI (OR 1.01, 95% CI 1.00-1.01, p=0.007) - in a study of transapical TAVI patients this was found to be the only predictive parameter for AKI (though again the investigators used the unmodified RIFLE criteria)14. However, increasing stage of chronic kidney disease was independently associated with a 1.5-fold increase in risk of AKI. Emboli in TAVI can be heralded by stroke –but there was association with the incidence of stroke and AKI to suggest that mechanism (OR 1.84, 95% CI 0.58-5.90, p=0.303). Interestingly the higher peak transaortic gradient was independently associated with lower risk of AKI. It is possible that this is a reflection of the LV function. Our study is likely not sufficiently powered to detect the effects of LV function but the incidence of poor LV function (i.e., LV ejection fraction ≤30%) was higher in the AKI group (15.7% versus 11.9% - though it did not reach statistical significance) and patients with poor LV function had lower transaortic peak gradients (50.5±20.6 vs. 74.5±24.0 mmHg).
Identification of patients more likely to be at risk of AKI may guide us in targeting some preventative therapies. In post-PCI patients this has included trials of prophylactic renal replacement therapy (with varying success)38,39, intravenous hydration and agents such as furosemide, mannitol and N-acetyl cysteine31,40,41. Preventative measures suggested to be beneficial in preventing AKI after cardiac surgery have included fenoldopam, vasodilators, atrial natriuretic peptide and brain natriuretic peptide42-44. These may provide possible strategies for reno-protection in these high-risk TAVI patients to be tested in future randomised studies. It should be understood however that preventative measures such as optimal hydration, minimising contrast and consideration of cessation of nephrotoxic medication should be routine in all our TAVI patients.
Limitations
The data for our study were gathered prospectively into a TAVI-specific patient database. However, the analyses were performed post hoc, using definitions devised after the initiation of data collection and are limited by the available parameters. The study, like all registry data, is therefore subject to the effects of possible confounders and bias in its design. The small size of the study means that differences in age and CKD for those with and without AKI may not have reached significance due to limited power.
Conclusion
Acute kidney injury is an important complication of TAVI and was observed in 35.9% of patients using the definition agreed by the Valve Academic Research Consortium. There was a significant relationship between AKI-as-defined and mortality at both 30 days and one year. Patients with pre-existing diabetes mellitus, peripheral vascular disease, higher stage of existing chronic kidney disease and those undergoing TAVI by an approach other than TF-TAVI are at greatest risk. Further investigation of reno-protective interventions is necessary to optimise the treatment of these patients, which has the potential to result in significant improvement in mortality following TAVI.
Conflict of interest statement
M. Thomas, C. P. Young, V. Bapat and S. Redwood are proctors for Edwards Lifesciences Inc. M. Thomas, S. Redwood and M.Z. Khawaja have received research funding from Edwards Lifesciences Inc. The remaining authors have no conflicts of interest to declare.

