Abstract
Aims: Acute kidney injury (AKI) is a strong but rather late predictor of mortality after transcatheter aortic valve implantation (TAVI). Early clinically useful markers for the detection of AKI and prediction of outcome are needed in order to control and improve management of periprocedural complications after TAVI. The aim of our study was to assess the predictive value of the Doppler-based renal resistance index (RRI), which correlates inversely with effective renal blood flow and creatinine clearance, for AKI in patients undergoing TAVI and to evaluate its association with paravalvular aortic regurgitation (PAR).
Methods and results: TAVI was performed with the Medtronic CoreValve prosthesis in 132 consecutive high-risk patients (mean logistic EuroSCORE: 30.3±18.2%). RRI, serum creatinine and cystatin C level were determined before, and 4 hrs, 24 hrs, 48 hrs, 72 hrs, and 7 days after TAVI. AKI occurred in 32/132 patients (24.2%). While serum creatinine and cystatin C levels decreased at first after TAVI (also in most patients developing AKI), the RRI increased significantly immediately after the procedure from 0.79±0.09 to 0.87±0.12 in patients developing AKI (p=0.003). A RRI >0.85 predicted post-interventional AKI with a sensitivity of 58% and specificity of 86%, and was superior to the serum creatinine level (p<0.001). In addition, an elevated RRI was significantly related to haemodynamic changes after TAVI and was associated with the occurrence of moderate/severe PAR (p<0.001).
Conclusions: Measurement of the Doppler-based RRI predicts risk for AKI and increased mortality rates at an early post-procedural time point and is related to the occurrence of more-than-mild paravalvular aortic regurgitation after TAVI.
Introduction
Transcatheter aortic valve implantation (TAVI) has emerged as an alternative to open heart surgery for patients with symptomatic severe aortic stenosis considered to be at very high or prohibitive operative risk1-10. However, in the currently treated patient population, which is characterised by severe comorbidities, the overall prognosis is significantly influenced by periprocedural complications. This includes the occurrence of acute kidney injury (AKI) in 12% to 28% of patients undergoing TAVI10-17. AKI has a significant impact on the prognosis of TAVI patients and is a powerful predictor of unfavourable early and late outcome, independent of whether renal function returns to baseline or not10,11,15-17. However, prevention and treatment of AKI is hampered by the absence of early predictors of renal function and the delayed increase of serum creatinine. Early detection of AKI might allow more precise targeting of therapeutic strategies18. Furthermore, the multifactorial pathophysiology of AKI in the setting of TAVI has not been completely elucidated as yet. Evidence is growing that periprocedural factors, such as the amount of contrast dye, red blood cell (RBC) transfusion, inflammation and also haemodynamic changes after implantation of transcatheter heart valves, might play a pivotal role10-17.
The Doppler-based renal resistance index (RRI) has been shown to correlate with serum creatinine levels and also reflects systemic haemodynamics19-22. Recent data suggest that renal RI might be a promising tool for the prediction of AKI in postoperative and critically ill patients23-26. We hypothesised that the Doppler-based RRI will help: 1) to detect patients at risk for AKI; 2) to detect haemodynamic disturbances after valve replacement; and 3) to foresee clinical course in TAVI patients.
Methods
PATIENT POPULATION
From January 2010 to December 2011, 132 consecutive patients with severe, symptomatic aortic stenosis at high risk for surgical aortic valve replacement (SAVR) underwent TAVI at our institution and were included into this prospective study after written informed consent was obtained. TAVI was performed with biplane fluoroscopy under local anaesthesia in combination with a sedative/analgesic treatment as described previously11. Patients with preprocedural chronic renal failure (CRF) received intravenous hydration and N-acetylcysteine before and after TAVI and were prophylactically treated with intravenous bicarbonate to prevent contrast-induced nephropathy. Visipaque 320 (GE Healthcare, Munich, Germany) was used as contrast medium in all patients with CRF.
The clinical endpoint of the study was the occurrence of AKI according to the definition of the Valve Academic Research Consortium (VARC-2): AKI was defined as an increase in serum creatinine to 150-200% (1.5-2.0x compared with baseline) or an increase of ≥0.3 mg/dL (≥26.4 μmol/L) for up to seven days after TAVI27. Chronic renal failure was defined as an eGFR ≤60 mL/min/1.73 m2.
The occurrence and severity of paravalvular aortic regurgitation (PAR) after TAVI was assessed by angiography, calculation of the aortic regurgitation index, and echocardiography after TAVI in accordance with the VARC-2 criteria. The evaluation of PAR was performed by a blinded echocardiographer, who did not attend the procedure.
Information pertaining to the cause of death was obtained from the referring cardiologist, treating hospital, and general practitioner charts. The study was approved by the local ethics committee of the University of Bonn.
DOPPLER-BASED DETERMINATION OF THE RENAL RESISTANCE INDEX
In all subjects, colour Doppler examination with measurement of the RRI was performed before, and 4 hrs, 24 hrs, 48 hrs, 72 hrs, and 7 days after TAVI with a Philips iE33 ultrasound scanner (Koninklijke Philips Electronics N.V., Amsterdam, The Netherlands) and a 2-5 MHz curved-array multifrequency transducer in supine position. Peak systolic velocity (Vmax [cm/sec]) and end-diastolic velocity (Vmin [cm/sec]) were obtained for the calculation of the dimensionless RRI values according to the following formula: resistance index=1−(Vmin/Vmax) (Figure 1). RRI was calculated as the average of four to six measurements both in representative interlobar and segmental arteries from the upper, middle, and lower third of both kidneys. The mean of the right and left mean RRI was calculated as total RRI. The Doppler angle was chosen as close to 0° as possible, and special care was taken not to compress the kidney and not to have the patient perform a Valsalva manoeuvre because both could increase and, therefore, confound the RRI value20,21. All measurements were performed by two independent cardiologists who were blinded to the corresponding biomarker levels.
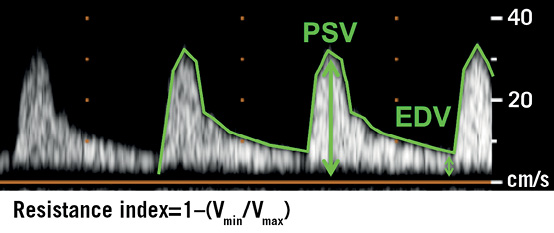
Figure 1. Calculation of the Doppler-based renal resistance index. Calculation of the dimensionless renal resistive index according to the formula: 1−(Vmin/Vmax). EDV: end-diastolic velocity; PSV: peak systolic velocity
LABORATORY METHODS
Serum creatinine and cystatin C levels were measured before, and 4 hrs, 24 hrs, 48 hrs, 72 hrs, and 7 days after TAVI (ECREA and CYSC Flex reagent cartridge, Dimension Vista®; Siemens Healthcare Diagnostics GmbH, Munich, Germany). Estimated glomerular filtration rate (eGFR) was calculated by the simplified Modification of Diet in Renal Disease (MDRD) formula.
STATISTICAL ANALYSIS
Data are presented as mean±standard deviation if normally distributed or as median and interquartile range (quartile 1/quartile 3) if not normally distributed. Categorical variables are given as frequencies and percentages. For continuous variables, a Student’s t-test was used for comparison between two groups. Pearson’s and Spearman’s correlation coefficients were used to establish associations. χ2 test was used for analysis of categorical variables.
Survival according to the RRI was determined using the Kaplan-Meier method. The log-rank test was used to determine statistical differences in terms of survival. For the RRI, a cut-off value for the prediction of one-year mortality after TAVI was calculated in ROC curve analysis (maximum sum out of sensitivity and specificity). We performed univariate and multivariate Cox regression analysis to examine the association of RRI and other clinical characteristics with one-year mortality. Because the event rate was not sufficient to support multivariate analysis with inclusion of all preprocedural and periprocedural mortality predictors in our study, such that a multivariate model would have been overfitted, we decided to focus on periprocedural mortality predictors only. Multiple linear regression analysis was applied to identify factors that were independently associated with the RRI.
The method of Bland and Altman was used for the assessment of inter-observer agreement in the first 50 consecutive patients. This showed no evidence of any systematic difference regarding inter-observer variability (κ=0.87; p<0.001). Intra-observer agreement for the average measures of the RRI was 0.96 (95% CI: 0.94-0.97) in the first 25 patients.
All tests were two-sided, and a p-value of <0.05 was considered statistically significant. Statistical analyses were conducted with PASW Statistics version 21.0.0.0.0 64-bit (IBM Corporation, Somers, NY, USA) and MedCalc version 11.1.1.0 (MedCalc Software, Mariakerke, Belgium).
The investigators initiated the study, had full access to and analysed the data, and wrote the manuscript. All authors support the data and analysis.
Results
BASELINE CHARACTERISTICS
TAVI was performed in 132 consecutive patients (mean age 80.9±6.6 years, 53.8% male gender) at high risk for SAVR as reflected by a mean STS (Society of Thoracic Surgeons) mortality score of 9.8±7.0% and a mean logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation) of 30.3±18.2%. Baseline characteristics are summarised in Table 1.
ACUTE KIDNEY INJURY AFTER TAVI
AKI occurred in 32 patients (24.2%) after TAVI, of whom 11 (8.3%) suffered from significant AKI (stage 2-3) (Figure 2). Patients with the development of AKI after TAVI were characterised by increased baseline serum creatinine levels (1.45 [1.09/1.84] mg/dL vs. 1.24 [0.99/1.53] mg/dL; p=0.08) and cystatin C levels (1.41 [1.09/1.80] mg/L vs. 1.21 [0.96/1.54] mg/L; p=0.049) (Table 1). AKI patients suffered more frequently from previous myocardial infarction (40.0% vs. 13.7%; p=0.001) and had a lower left ventricular ejection fraction (LVEF) (40.4±15.3% vs. 47.7±14.4%; p=0.006). The pre-interventional RRI was not different in patients with AKI after TAVI (0.79±0.09 vs. 0.78±0.08; p=0.67). At baseline, a weak correlation of RRI values with serum creatinine (r=0.23; p=0.001) and cystatin C levels (r=0.25; p=0.005) was found.
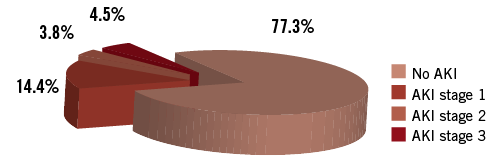
Figure 2. Rates of acute kidney injury according to VARC-2 definitions. Significant AKI (stage 2-3) occurred in 11 patients (8.3%) after TAVI.
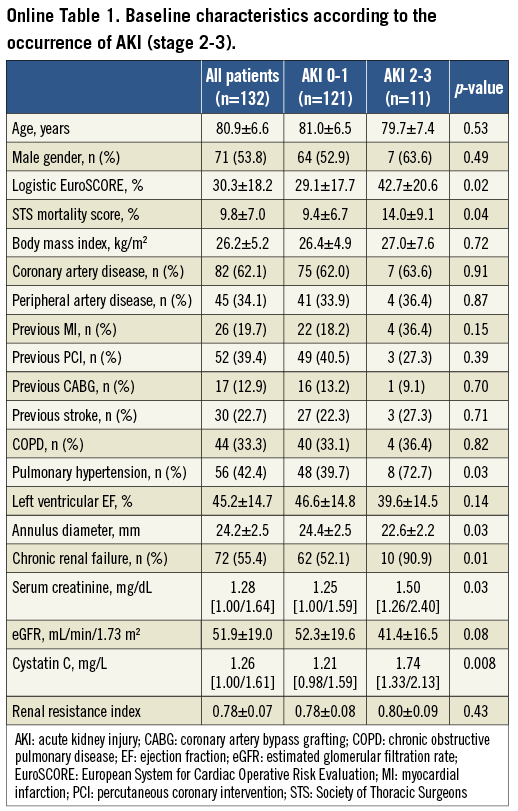
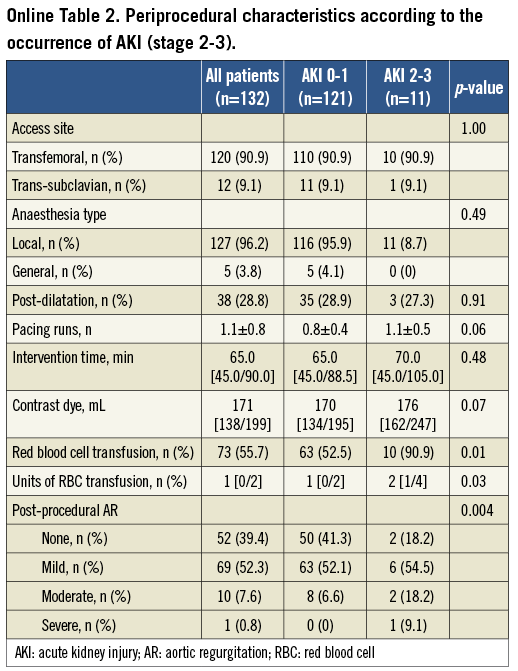
The development of AKI was associated with RBC transfusion (80.0% vs. 48.5%; p=0.002), major vascular complications (13.3% vs. 3.9%; p=0.058), the amount of contrast dye (186 [163/234] mL vs. 169 [133/192] mL; p=0.01), and moderate/severe PAR after TAVI (16.6% vs. 5.9%; p=0.04) (Table 2). In addition, we noticed a significant relationship of AKI with the incidence of a systemic inflammatory response syndrome (SIRS) after the procedure (63.3% vs. 26.0%; p<0.001). Baseline and procedural characteristics according to the occurrence of significant AKI (stage 2-3) compared to non-significant AKI (no AKI, AKI stage 1) are summarised in Online Table 1 and Online Table 2.
RENAL RESISTANCE INDEX AND RENAL BIOMARKERS AFTER TAVI
In patients developing AKI, a significant increase of the RRI baseline values (from 0.79±0.09 to 0.87±0.12; p=0.003) was observed immediately at four hours after the procedure (Figure 3A). In AKI patients, serum creatinine levels (Figure 3B) did not show a significant increase until 48 hours (from 1.41 [1.12/1.78] mg/dL to 1.71 [1.30/2.03] mg/dL; p=0.001) and cystatin C levels (Figure 3C) not until 72 hours after TAVI (from 1.40 [1.08/1.76] mg/L to 1.57 (1.21/2.10) mg/L; p=0.004), respectively. The maximum correlation of the RRI with serum creatinine levels (r=0.45; p<0.001) and with cystatin C levels (r=0.44; p<0.001) was seen after 72 hours.
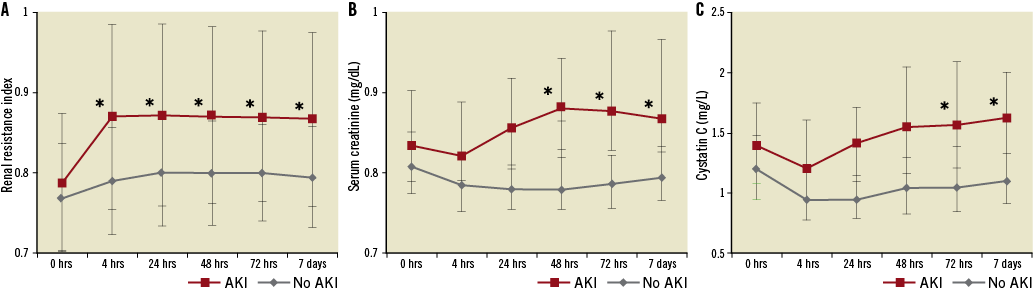
Figure 3. Renal resistance index, serum creatinine and cystatin C after TAVI. Time course of the renal resistance index (A), serum creatinine level (mg/dL) (B), and cystatin C level (mg/L) (C) before, and 4 hrs, 24 hrs, 48 hrs, 72 hrs, and 7 days after TAVI according to the occurrence of acute kidney injury. Asterisks (*) indicate a significant increase compared to baseline values before TAVI, p<0.01.
In ROC curve analysis, we calculated a cut-off value of the RRI for the prediction of AKI. At four hours after TAVI, a RRI >0.85 was able to predict post-interventional AKI with a sensitivity of 58% and a specificity of 86%, and was superior to the prediction of AKI by a serum creatinine increase ≥0.3 mg/dL (area under the curve [AUC]: 0.73, 95% CI: 0.61-0.85 vs. AUC: 0.56, 95% CI: 0.44-0.68; p<0.001).
RRI AND CLINICAL OUTCOMES
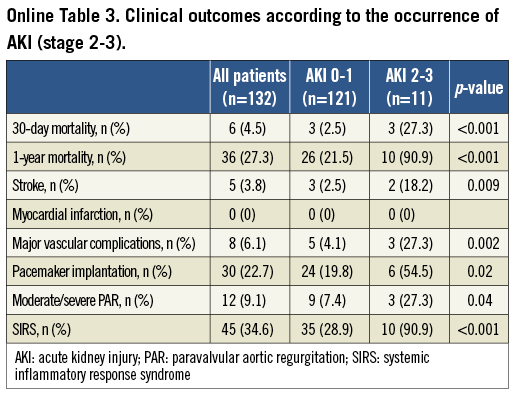
The 30-day mortality rate was 4.5% (6/132 patients), and the cumulative one-year mortality rate was 27.3% (36/132 patients) (Table 3). The development of AKI was a strong predictor of one-year mortality (56.7% vs. 18.6%; p<0.001) (Figure 4A). The occurrence of significant AKI (stage 2-3) was associated with an even worse outcome (one-year mortality: 90.9% vs. 21.5%; p<0.001) (Figure 4B, Online Table 3). A post-procedural RRI >0.85, which was found in 30 of 132 patients (22.7%) after TAVI, was strongly associated with one-year mortality (66.7% vs. 15.7%; p<0.001) (Figure 5). In univariate Cox regression analysis, patients with an RRI >0.85 had a sevenfold increased one-year mortality risk (hazard ratio [HR] 7.0, 95% CI: 3.6-13.6; p<0.001) during follow-up compared to other patients. In multivariate analysis after adjustment for the occurrence of AKI, major vascular complications, SIRS and moderate/severe PAR, a RRI >0.85 remained as an independent predictor and was still associated with a more than threefold increased one-year mortality risk (HR 3.2, 95% CI: 1.4-7.3; p=0.005) (Table 4).
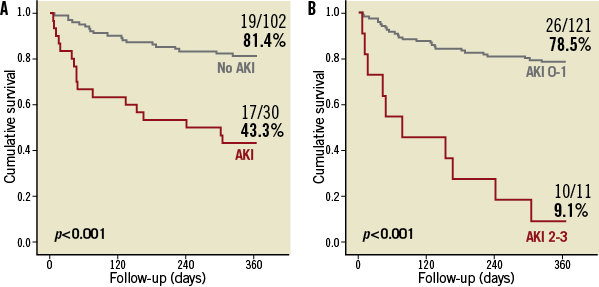
Figure 4. One-year survival according to the occurrence of acute kidney injury. Patients were stratified according to the occurrence of acute kidney injury (AKI) (A) and significant AKI (stage 2-3) (B) during the first seven days after TAVI.
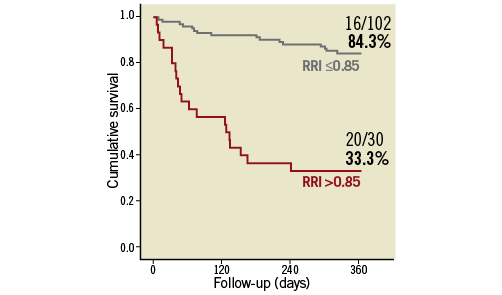
Figure 5. One-year survival according to the renal resistance index. Survival rate according to the ROC-curve-based cut-off value of the resistance index (RRI ≤0.85 vs. RRI >0.85), which was determined four hours after transcatheter aortic valve implantation.
RRI AND PARAVALVULAR AORTIC REGURGITATION AFTER TAVI
In multivariate regression analysis, a strong association was shown between the RRI and the severity of PAR (p<0.001), which was in turn also significantly related to AKI (p=0.017). Furthermore, in this multivariate analysis, the RRI was related to the serum creatinine level (p=0.022), the occurrence of acute kidney injury (p=0.001) and SIRS (p=0.006), and the prevalence of coronary artery disease (p=0.028) (Table 5).
The post-procedural RRI differed significantly with increasing severity of PAR in patients with unchanged renal function after TAVI, and was significantly increased in patients suffering from moderate/severe PAR compared to patients without or with only mild PAR (0.95±0.08 vs. 0.78±0.05; p<0.001). In addition, the RRI strongly correlated with the recently published AR Index29 (r= –0.454; p<0.001). Even in AKI patients, the RRI was associated with the degree of PAR, and was significantly elevated in patients with significant PAR (0.97±0.07 vs. 0.85±0.11; p=0.017) (Figure 6).
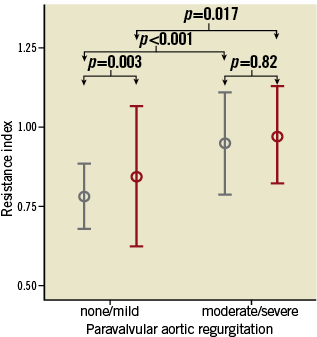
Figure 6. Association of renal resistance index and paravalvular aortic regurgitation. Association of the dimensionless renal resistance index (mean±2 standard deviation) with the severity of paravalvular aortic regurgitation (none/mild vs. moderate/severe). No AKI (grey bars), AKI (red bars). AKI: acute kidney injury
Discussion
In this prospective study of 132 consecutive patients, we evaluated the value of the Doppler-based RRI, which is an integration of arterial compliance, pulsatility, and peripheral renal resistance, for patients after TAVI. The RRI is able to predict AKI in the clinically crucial, early post-procedural hours immediately following TAVI - earlier than the established serum biomarkers creatinine and cystatin C, which both decrease during the first 24 hrs after the procedure – even in the majority of patients developing AKI. In addition, the RRI was significantly associated with the occurrence of more-than-mild PAR and correlated with the invasively measured AR Index. Therefore, the RRI might be helpful as part of a multimodal approach for the precise quantification of PAR and as a non-invasive parameter even after the procedure. The RRI also strongly predicted one-year mortality after TAVI as surrogate for a combination of two major determinants of mortality after the TAVI procedure: AKI and PAR.
PATHOPHYSIOLOGY OF AKI AFTER TAVI
Emerging data suggest that AKI has a significant impact on patient morbidity, length of hospital stay, and mortality after TAVI10-17. AKI is observed with an incidence of 12-28% in patients undergoing TAVI and accounts for a 30-day mortality rate of 23-25% and a one-year mortality of 55-60%, respectively10,11,15-17. The pathophysiology of AKI after TAVI seems to be multifactorial: nephrotoxic, inflammatory, atheroembolic and haemodynamic factors are involved and overlap each other, leading to an abrupt worsening of renal function17,28. In addition to patient-related variables such as CRF, history of previous myocardial infarction, and impaired LVEF, recent data suggested periprocedural variables such as major vascular complications or bleeding events, more-than-mild PAR, and RBC transfusion during the first 72 hours as independent predictors of AKI10-13,15-17.
RRI COMPARED TO ESTABLISHED RENAL BIOMARKERS
Renal hypoperfusion and subsequent ischaemic injury are considered to play a significant role in the pathogenesis of AKI. They can arise from either a decrease in renal perfusion pressure or an increase in renal vascular resistance. Importantly, renal vascular resistance increases, even when systemic vascular resistance is low, as in SIRS or sepsis. The RRI has recently been suggested for assessing changes in renal perfusion in cardiac surgery patients22,23 and for predicting AKI in critically ill patients admitted to the intensive care unit24-26,30. Many factors influence the RRI and have to be taken into account when interpreting RRI values in cardiac surgery patients, such as stiffness of the aorta and observer-dependent factors. Rather than being considered specific markers of renal damage, intrarenal RRIs are an integration of arterial compliance, arterial pulsatility, and peripheral resistance20,31. In line with this concept of considering ultrasound resistance indices as systemic vascular rather than local renal markers, we found an association of RRI values with the occurrence of more-than-mild PAR and a correlation with the AR Index.
In patients suffering from AKI, serum creatinine elevation, which is the currently accepted “gold standard” to define AKI, does not occur until days after renal tubular injury has begun, as GFR eventually becomes impaired as a consequence of tubular obstruction and tubuloglomerular feedback17,32. Similarly in cardiac surgery, it is commonly observed that serum creatinine levels decrease in the immediate postoperative period, even in those patients who develop severe AKI requiring renal replacement therapy33. In our study, this phenomenon was confirmed in TAVI patients. However, the RRI was able to identify patients developing AKI immediately after TAVI. For the time being, strategies to treat AKI in TAVI patients remain an important challenge. Patients with increased RRI need special attention on the intensive care unit and might profit from an intensive fluid management. In all of these patients, the severity of PAR should be reassessed to identify patients with clinically significant PAR, which also influences the RRI after TAVI, as these patients might benefit from corrective measures to reduce PAR. Data about prophylactic haemofiltration in patients with AKI after cardiac surgery are empirical and cannot be extrapolated to TAVI patients.
RRI AND PARAVALVULAR AORTIC REGURGITATION
It has been hypothesised that, in the presence of significant AR, the RRI as an integration of arterial compliance, pulsatility, and peripheral resistance might not accurately reflect renal resistance, as the renal artery diastolic velocity would be significantly reduced and this could result in an inaccurate impression of reduced renal blood flow from increased renal vascular resistance34,35. Our study is the first to confirm this hypothesis in man: due to the reduced systemic diastolic blood pressure and, thereby, increased pulse pressure in patients with moderate/severe PAR, the RRI values were significantly increased in our study. In all of the six patients with significant PAR after TAVI who developed AKI, holodiastolic flow reversal in the aorta was observed. Hence, the RRI values in these patients might also mirror reduced diastolic renal blood flow with consecutive impairment of creatinine clearance and subsequent AKI as a consequence of significant PAR, finally culminating in an acute cardiorenal syndrome.
The Doppler-based RRI, which is easily available during and after the procedure, mirrors clinically relevant haemodynamic alterations due to paravalvular leakage after TAVI and also correlates with the recently introduced AR Index29. In addition to imaging modalities such as echocardiography, the RRI might be useful to quantify the degree of PAR as a non-invasive parameter in patients with borderline AR, especially during follow-up. The RRI might be an important hint to close the gap between renal function and mortality after TAVI, as it sheds light on the direct association of diastolic renal perfusion, renal function, and the occurrence of AKI with paravalvular aortic regurgitation after the procedure. In the TAVI patients of our study cohort, a post-procedural elevation of the RRI above 0.85 was associated with the development of AKI and/or the occurrence of moderate/severe PAR. This might have synergistically affected mortality, since both have been shown to be strong predictors of an adverse outcome after TAVI10,11,29.
Study limitations
Our study is a hypothesis-generating pilot study that allows identification of clinical criteria important to optimise patient selection and post-procedural management. Results need to be confirmed in larger trials.
Conclusions
Measurement of the Doppler-based renal resistance index predicts risk for acute kidney injury and increased mortality rates at an early post-procedural time point. In addition, the RRI is related to the occurrence of moderate/severe paravalvular aortic regurgitation after TAVI.
Conflict of interest statement
E. Grube is a proctor for Medtronic (CoreValve). The other authors have no conflicts of interest to declare.