Abstract
Aims: We aimed to assess whether the RenalGuard™ System is effective in preventing acute kidney injury (AKI) following transcatheter aortic valve implantation (TAVI).
Methods and results: Forty-eight consecutive patients with chronic kidney disease (CKD) scheduled for TAVI were assigned to: 1) hydration with sodium bicarbonate solution (Control group), or 2) hydration with RenalGuard Therapy® (RenalGuard group). Hypotension was defined as periprocedural mean blood pressure <55 mmHg. The primary endpoint was the occurrence of AKI (i.e., an increase of ≥0.3 mg/dL in the serum creatinine concentration at seven days). AKI occurred in 10/26 (38.5%) patients in the Control group and in 1/22 (4.5%) patients in the RenalGuard group (p=0.005, odds ratio [OR] 0.076, 95% confidence interval [CI]: 0.009-0.66). RenalGuard Therapy protected against AKI (OR 0.71, 95% CI: 0.07-0.775, p=0.026), whereas post-procedural hypotension (OR 3.88, 95% CI: 1.06-14.24, p=0.040), and contrast media volume (OR 3.65, 95% CI: 1.15-5.75, p=0.043) increased the risk of AKI.
Conclusions: This non-randomised pilot study suggests that RenalGuard Therapy may be effective in preventing AKI in CKD patients undergoing TAVI.
Introduction
Acute kidney injury (AKI) is a common complication of transcatheter aortic valve implantation (TAVI)1-3. RenalGuard Therapy® (RenalGuard Solutions Inc., Milford, MA, USA) is effective in preventing contrast-induced AKI (CI-AKI)4. We herein report our experience with the RenalGuard System™ (RenalGuard Solutions Inc.) to prevent AKI in patients with chronic kidney disease (CKD) undergoing TAVI.
Methods
This was a pilot, prospective, non-randomised study. From January 2009 to December 2014 all consecutive patients with CKD scheduled for TAVI through the femoral approach were included in the study. In all instances iodixanol was injected. Contrast media (CM) volume >3 times the estimated glomerular filtration rate (eGFR) was considered high5. The study was approved by our ethics committee and all patients signed written informed consent.
Patients were assigned to one of the following groups: 1) Control group, or 2) the RenalGuard group. Criteria for treating the patients with the RenalGuard System were: a) eGFR ≤30 mL/min/1.73 m2, or b) a predicted risk for AKI ≥50%6,7. Patients allocated to the Control group received 154 mEq/L of sodium bicarbonate in dextrose and H2O plus N-acetylcysteine (NAC) at a high dose4. In the RenalGuard group, hydration was carried out with normal saline using the RenalGuard System in order to achieve an optimal urine flow of ≥300 mL/hr4.
The CoreValve ReValving™ prosthesis (Medtronic, Minneapolis, MN, USA) was implanted in all instances through the femoral approach. Hypotension was defined as mean blood pressure <55 mmHg at any time during or within the first 24 hours after the procedure, regardless of the need for vasopressor support8. The primary outcome measure was the development of AKI, defined as an increase in serum creatinine (sCr) concentration ≥0.3 mg/dL or ≥1.5-1.9 times above the baseline within seven days after the administration of contrast media9. Secondary endpoints and statistical analysis methods are reported in the Appendix.
Results
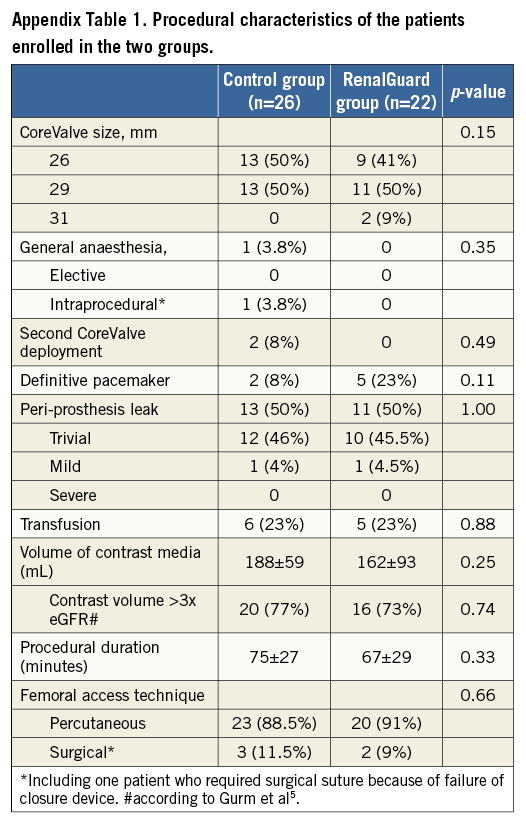
The characteristics of the patients in the two groups are reported in Table 1. Procedural details are reported in Appendix Table 1. The CM volume was similar in the two groups (RenalGuard group 188±59 mL; Control group 162±93 mL, p=0.25). Intraprocedural hypotension occurred in 17/26 (65.5%) in the Control group and in 13/22 (59%) in the RenalGuard group (p=0.76). Transfusion was required in six (23%) patients in the Control group and in five patients (23%) in the RenalGuard group (p=0.88). AKI occurred in 10/26 (38.5%) patients in the Control group and in 1/22 (4.5%) in the RenalGuard group (p=0.005; OR 0.076, 95% CI: 0.009-0.66) (Figure 1). In-hospital renal failure requiring dialysis occurred in two patients in the Control group (8%) versus none in the RenalGuard group (p=0.49). RenalGuard Therapy protected against AKI, whereas post-procedural hypotension and contrast media volume increased the risk of AKI (Table 2). Secondary endpoints are reported in the Appendix.
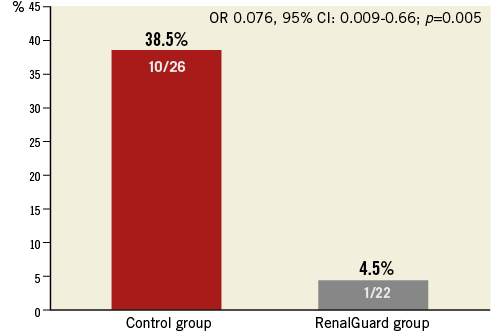
Figure 1. Incidence of contrast-induced acute kidney injury.
Discussion
The present pilot study suggests that RenalGuard Therapy is effective in preventing AKI in patients with CKD undergoing TAVI. The RenalGuard System enables the achievement of high urine output safely by maintaining the intravascular volume and minimising the risk of overhydration or underhydration4. The pathogenesis of AKI following TAVI encompasses “contrast-induced” and “contrast-independent” mechanisms. In the present study, post-procedural hypotension and CM volume represented independent predictors of AKI. A graded relationship between the length of time spent with a mean blood pressure (MAP) <55 mmHg and AKI has been reported8. As such, optimising perioperative haemodynamics may mitigate this complication. Although conflicting data have been reported1, our result highlights the importance of limiting the CM volume in this high-risk population2.
Study limitations
The limitations of the study are: 1) the small sample size, 2) the non-randomised design, and 3) the treatment of the Control group with sodium bicarbonate and NAC, which is actually not recommended10. A large randomised, multicentre trial comparing RenalGuard Therapy with the recommended hydration regimen (normal saline) is therefore necessary.
Conclusions
RenalGuard Therapy seems to be effective for AKI prevention in CKD patients undergoing TAVI.
| Impact on daily practice Acute kidney injury (AKI) is a frequent complication of transcatheter aortic valve implantation (TAVI) and may occur because of a combination of “contrast-induced” and “contrast-independent” (such as hypotension and transfusion) mechanisms. The RenalGuard Therapy has been reported to be an effective renoprotective strategy for contrast-induced AKI prevention. The present study supports the finding that the RenalGuard Therapy is more effective than a conventional hydration regimen in preventing AKI in patients with chronic kidney disease undergoing TAVI. Therefore, in daily practice we should include the RenalGuard System in our armamentarium for AKI prevention after TAVI. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
SUPPLEMENTARY DATA
Appendix. Methods and results details
METHODS
SECONDARY STUDY ENDPOINTS
1) An increase in sCr concentration ≥25% and ≥0.5 mg/dL.
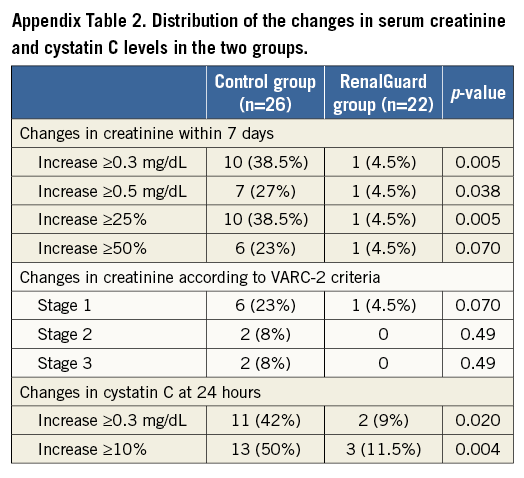
2) The severity of AKI as follows: Stage 1, a sCr increase ≥0.3 mg/dL or ≥1.5-1.9 times from baseline; Stage 2, a sCr increase ≥2.0-2.9 times from baseline; and Stage 3, a sCr increase ≥3.0 times from baseline or the need for dialysis.
3) Changes in the serum cystatin C (sCyC) concentration at 24 and 48 hours after contrast media exposure.
STATISTICAL ANALYSIS
Continuous variables are given as mean±1 standard deviation or median and IQR, when appropriate. The Student’s t-test and the non-parametric Mann-Whitney tests were used to determine differences between mean values for normally and abnormally distributed variables, respectively. Categorical variables were reported as percentages and were analysed by the Fisher’s exact test, as appropriate. Linear regression was performed to provide odds ratios (OR) with 95% confidence intervals (CI) and adjustment for selected risk factors (treatment group, post-procedural hypotension, contrast media volume, and transfusion). To correct for multiple testing, 100 bootstrap iterations were computed. A probability level <0.05 was considered significant throughout the analysis. Data were analysed with SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
HYPOTENSION
Duration of intraprocedural hypotension was 7.5 (3.5-19) minutes in the Control group and 6 (5-10) minutes in the RenalGuard group (p=1.00). Post-procedural hypotension occurred in 13/26 (50%) patients in the Control group and in 7/22 (32%) patients in the RenalGuard group (p=0.25). Duration of post-procedural hypotension was 90 (45-120) minutes in the Control group and 90 (44-145) minutes in the RenalGuard group (p=0.78).
HYDRATION VOLUME AND URINE VOLUME
In the RenalGuard group, we observed highly accurate, temporally matched fluid replacement during the treatment (Appendix Figure 1A), and the mean urine flow was 340±169 mL/hr (Appendix Figure 1B). Total hydration volume was higher in the RenalGuard group (2,728±938 mL versus 1,575±246 mL; p<0.001). Total urine volume was higher in the RenalGuard group at both 24 hours (2,709±1,296 mL versus 1,551±550 mL; p<0.001) and 48 hours (2,570±1,130 mL versus 1,634±922 mL; p=0.002). Periprocedural acute pulmonary oedema occurred in only one patient in the Control group.
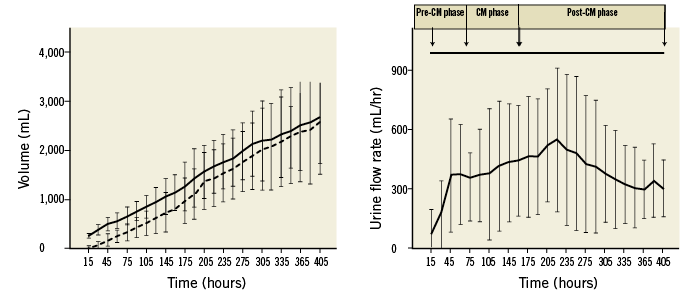
Appendix Figure 1. RenalGuard Therapy (mL/hr). A) Temporally matched fluid replacement during treatment by using the RenalGuard System (continuous line=infusion; dashed line=urine). B) Mean urine flow in the RenalGuard group. Urine output (mL/hr) was recorded every 15 minutes during RenalGuard Therapy. CM phase: contrast media exposure or intraprocedural time; Post-CM phase: post-contrast media or post-procedural time; Pre-CM phase: pre-contrast media exposure or pre-procedural time
AKI
Serum creatinine increased significantly more in the Control group than in the RenalGuard group (p=0.014; F=4.73 by repeated measure of variance ANOVA model) (Appendix Figure 2). In-hospital renal failure requiring dialysis occurred in two patients in the Control group (8%) versus none in the RenalGuard group (p=0.49). The distribution of different cut-offs of sCr increase at seven days, AKI severity and sCyC kinetic are reported in Appendix Table 2 and Appendix Figure 3. The intraprocedural hypotension rate was similar in the AKI group and the No-AKI group (8/11 [70%] versus 20/35 [57%]; p=0.48). Post-procedural hypotension occurred in 9/11 (82%) patients in the AKI group versus 10/35 (31%) patients in the No-AKI group (OR 11.25, 95% CI: 2.06-61.50, p=0.002). The transfusion rate was higher in the AKI group than in the No-AKI group (6/11 [54.5%] versus 5/35 [14.5%], OR 7.2, 95% CI: 1.57-32.86, p=0.006).
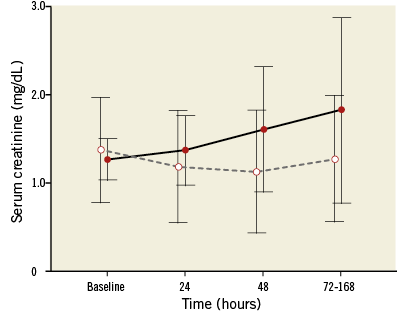
Appendix Figure 2. Serum creatinine kinetic. Serum creatinine concentration (median and interquartile range) at baseline, 24 hrs, 48 hrs and peak within seven days after contrast media administration in the Control group (continuous line) and in the RenalGuard group (dashed line); p=0.014; F=4.73 by repeated measure of variance ANOVA model.
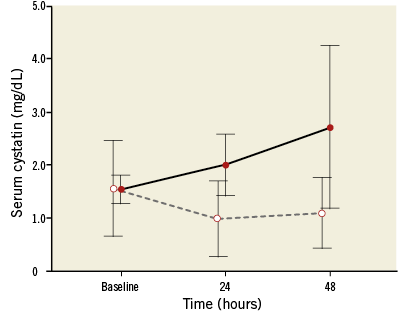
Appendix Figure 3. Serum cystatin C kinetic. Serum cystatin C concentration (median and interquartile range) at baseline, 24 and 48 hours after contrast media administration in the Control group (continuous line) and in the RenalGuard group (dashed line); p=0.006; F=6.68 by repeated measure of variance ANOVA model.