Abstract
Aims: Contrast-induced nephropathy (CIN) is a frequent and potentially harmful complication of percutaneous coronary interventions (PCI), especially in the setting of ST-elevation myocardial infarction (STEMI). We tested the efficacy of a sodium bicarbonate (SB)-based hydration in urgent PCI for STEMI.
Methods and results: From June 2009 to September 2010, 262 consecutive STEMI patients undergoing urgent PCI were prospectively enrolled and treated by SB-based hydration (154 mEq/L at 3 ml Kg–1 for one hour followed by 1 ml Kg–1 for six hours) (group A). As controls, 262 consecutive STEMI patients receiving 0.9% saline hydration (1 ml Kg–1 for 24 hours) before June 2009 were retrospectively enrolled (group B). Both groups received high-dose N-acetylcysteine (NAC). The primary endpoint was the composite of in-hospital death, need for dialysis and CIN (≥25% increase in serum creatinine at 48 hours). The two groups were comparable for baseline clinical and procedural characteristics, for Mehran risk score and baseline estimated glomerular filtration rate. The primary combined endpoint was significantly reduced in group A as compared to group B (9.2 vs. 18.7%, p=0.023) with a number needed to treat (NNT) of 11. Specifically, a significant reduction of both in-hospital death (2.3 vs. 6.1%, p=0.049, NNT 27) and CIN (8.0 vs. 14.1%, p=0.03, NNT 17) was observed, with no difference in the need for dialysis.Conclusions: Our data indicate that hydration with sodium bicarbonate in addition to high-dose NAC in the setting of urgent PCI for STEMI is associated with a net clinical benefit.
Abbreviations
CIN: contrast-induced nephropathy
eGFR: estimated glomerular filtration rate
MRS: Mehran risk score
NAC: N-acetylcysteine
NNT: number needed to treat
PCI: percutaneous coronary intervention
SB: sodium bicarbonate
sCr: serum creatinine
STEMI: ST-segment elevation myocardial infarction
VCCR: volume to creatine clearance ratio
Introduction
Contrast-induced nephropathy (CIN) is a relatively frequent complication of coronary angiography and cardiovascular interventions, accounting for about 10% of all causes of hospital-acquired renal failure1,2. Usually defined as the new onset or exacerbation of renal dysfunction after contrast administration in the absence of other causes, CIN prolongs in-hospital stay, even when renal dysfunction is transient, and represents a powerful predictor of poor early and late outcome3-5. Several clinical and procedural risk factors, including previously impaired renal function, advanced age, diabetes mellitus, congestive heart failure and the volume of contrast medium administered, have been identified as predictors of CIN6,7.
Primary percutaneous coronary interventions (PCI) in the setting of ST-segment elevation myocardial infarction (STEMI) represent a situation at high risk of CIN. Specifically, the reported incidence of CIN following primary PCI is considerably higher than that observed following elective procedures8. This increased risk is generally attributed to systemic hypoperfusion due to left ventricular (LV) dysfunction, to the larger volume of contrast medium and, more importantly, to the difficulty in providing an adequate hydration prior to the procedure.
The generation of reactive oxygen species is a recognised pathophysiological mechanism of CIN9. In this regard, N-acetylcysteine (NAC) and sodium bicarbonate (SB) have been proposed as potent antioxidant strategies in the field of nephroprotection10,11. In the setting of STEMI, high-dose NAC has been shown to reduce the occurrence of CIN, and also to lead to a significant decrease in mortality12. Conversely, volume supplementation with SB, which has previously been shown to reduce CIN in elective procedures13, has not been adequately tested in primary or rescue PCI, especially in combination with high-dose NAC. The aim of this study was to compare the clinical efficacy of the combination of SB and high-dose NAC in a prospectively-enrolled cohort of all-comers STEMI patients with an historical cohort of STEMI patients receiving standard saline hydration plus high-dose NAC.
Methods
STUDY POPULATION
The “BIcarbonato e N-Acetilcisteina nell’infaRto mIocardico acutO” (BINARIO) study is a non-sponsored registry specifically designed to test the efficacy of SB in addition to high-dose NAC on mortality and renal function in the setting of urgent PCI for STEMI. With this aim, from June 1st 2009 to September 30th 2010 all consecutive patients with STEMI undergoing primary or rescue PCI were prospectively enrolled and treated with SB-based hydration plus high-dose NAC. As controls, the same number of consecutive patients undergoing primary or rescue PCI for STEMI before May 31st 2009 and treated with saline hydration plus high-dose NAC were retrospectively enrolled. Exclusion criteria included age >90 years, end-stage renal failure on dialysis, need of urgent cardiac surgery as coronary revascularisation instead of urgent PCI, known allergy to iodinated contrast or NAC, NAC unavailability and pregnancy or lactation (Figure 1). Cardiogenic shock was defined as prolonged hypotension (systolic blood pressure <90 mmHg for at least 30 min) requiring inotropic support medication and/or intra-aortic balloon pump to maintain a systolic blood pressure of >90 mmHg.
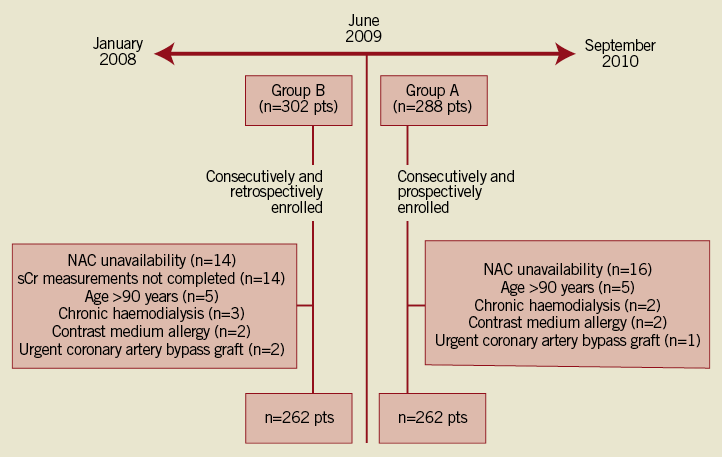
Figure 1. Study profile: diagram showing the flow of study patients. NAC: N-acetylcysteine; sCr: serum creatinine
The study protocol was reviewed and approved by the local ethics committee. All patients receiving SB-based hydration gave written informed consent before entry in the study. The study was registered at Clinicaltrials.gov as NCT01218178.
STUDY PROTOCOL
Patients treated by SB plus NAC received an infusion of 154 mEq/L SB (obtained adding 77 ml of 1,000 mEq/l SB to 433 ml of 5% glucose solution) at a rate of 3 ml kg–1 for one hour followed by 1 ml kg–1 for six hours (group A)12. Patients treated with saline hydration received 0.9% isotonic saline at a rate of 1 ml kg–1 h–1 for 24 hours (or 0.5 ml kg–1 h–1 in case of overt heart failure) (group B). Patients of both groups were also treated with high-dose NAC according to Marenzi et al (1,200 mg intravenously in bolus before the procedure followed by 1,200 mg per os b.i.d. for the following 48 hours)13. During the intravenous administration of SB or isotonic saline, further volume supplementation was discouraged. Oral intake of water was liberal but it never exceeded one litre/day according to our standard practice. Nurses were responsible for the application of the prescribed therapies ticking a dedicated check box on the patient’s drug sheet. During PCI, patients continued the assigned therapy.
The use of inotropic drugs, beta-blockers (metoprolol from 25-50 mg b.i.d. when LV function is preserved and carvedilol 3.125 mg b.i.d. titrated to 12.5-25 mg b.i.d. when LV ejection fraction is <40%), renin-angiotensin system antagonists (ramipril 2.5-5 mg/day or valsartan 40-80 mg/day) and diuretics was left to the discretion of interventional and coronary care unit cardiologists, according to current ESC STEMI guidelines14. Left ventricular function was evaluated by echocardiography in all patients within 24 hours following admission. Serum creatinine (sCr) and cardiac Troponin T (cTnT) were serially measured in all patients at emergency room admission and then every 12 hours until 48 hours after PCI. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) study group equation15. Percutaneous coronary interventions were performed according to our local standard practice and described in detail in the online supplementary material. Low-osmolality nonionic contrast media iomeprol and iopamidol (Iomeron 350 and Iopamiro 370; Bracco S.p.a, Milan, Italy) with 350-370 mg/ml of iodine content were used in all cases.
ENDPOINTS
The primary endpoint was the composite of in-hospital death, need for dialysis and CIN. CIN was defined as a relative increase of ≥25% in sCr concentration over the baseline value at 48 hours after PCI. Dialysis was undertaken in patients with oligoanuria (urine output <20 ml/h for 24 h) despite the administration of more than 1 g intravenous furosemide and presence of volume overload. When more than one event occurred in the same patient (e.g., death and CIN), the primary endpoint was counted as a single event. The following in-hospital secondary endpoints were also assessed: 1) the individual components of the primary endpoint; 2) other conventional definitions of CIN, including absolute increase in sCr ≥0.5 mg/dl from baseline, or relative increase ≥50% in sCr from baseline, or relative decrease ≥25% in eGFR from baseline value at 48 hours after PCI; 3) the change in sCr and eGFR at 48 hours.
A pre-specified subgroup analysis according to gender and categories of patients at higher risk of developing CIN, such as age ≥75 years, baseline eGFR ≤60 ml/min, presence of diabetes, volume of administered contrast media, volume to creatinine clearance ratio (VCCR) and Mehran risk score (MRS) was also performed. VCCR was calculated by dividing the administered volume of contrast by the patient’s eGFR16. As previously shown, a VCCR >3.7 identifies a population at high risk of CIN. MRS is a validated weighted scoring system for prediction of CIN based on clinical data collected before and during PCI, including hypotension (five points), intra-aortic balloon pump (five points), history of New York Heart Association (NYHA) Class III/IV congestive heart failure (five points), eGFR (<20 mL·min–1·1.73 m–2: 6 points; 20-40 mL·min–1·1.73 m–2: four points; 40-60 mL·min–1·1.73 m–2: two points), diabetes mellitus (three points), age >75 years (five points), anaemia (three points) and contrast volume (one point for each 100 cc)17. MRS allows the identification of four groups at increasing risk of developing CIN: scores ≤5 (low risk), 6-10 (moderate risk), 11-15 (high risk) and ≥16 (very high risk).
STATISTICAL ANALYSIS
The sample size of the study was calculated assuming a rate of the composite primary endpoint of about 15% in group B, based on the rate previously reported by Marenzi et al12 in patients undergoing primary PCI, and hypothesising a reduction to below 10% in group A. Power calculation indicated a sample size of about 250 patients for each arm to detect a statistically significant difference between the two groups with an 80% power and a type I error of 0.05. Normality was tested by the D’Agostino-Pearson test. Categorical variables were expressed as percentages and analysed by the two-tailed Fisher’s exact test. Continuous variables were expressed as mean±SD and compared with the t-test or with the nonparametric Wilcoxon or Mann-Whitney U test, as appropriate. Number needed to treat (NNT) was calculated for the primary composite endpoint and for its individual components. All statistical analyses were performed with the Statistical Package for the Social Sciences version 19.0 (SPSS Inc., Chicago, IL, USA). A two-sided p-value of 0.05 was considered to be significant.
Results
STUDY POPULATION
From June 1st 2009, 288 patients were consecutively screened. Twenty-six patients were excluded for NAC unavailability (n=16), age >90 years (n=5), chronic haemodialysis (n=2), contrast-medium allergy (n=2) or urgent coronary artery bypass grafting (n=1) (Figure 1). This led to a final cohort of 262 prospectively-enrolled patients treated with SB-based hydration (group A). 262 consecutive retrospectively-enrolled STEMI patients treated with saline hydration before June 1st 2009 then constituted the historical cohort of the study (group B) (Figure 1). Of note, the time of enrolment for the two groups was similar (14 vs. 16 months, respectively).
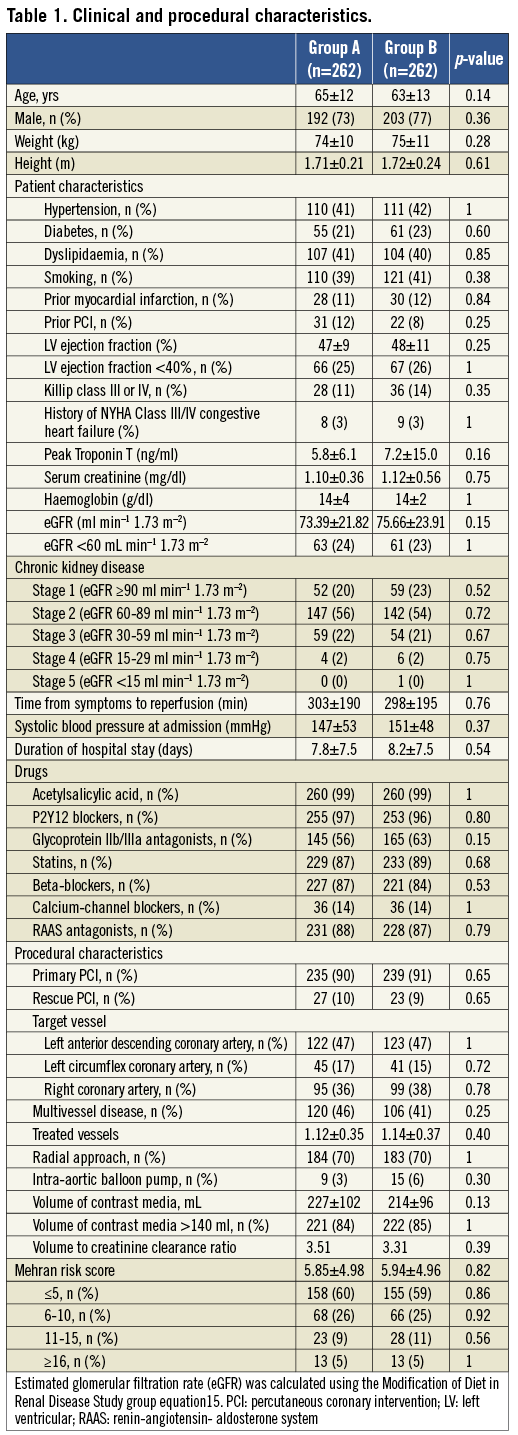
Baseline demographic, clinical and procedural characteristics of the study population are shown in Table 1. The two groups did not differ in terms of age or gender. Similarly, the prevalence of cardiovascular risk factors, clinical history or presentation, administered therapy and cTnT peak release were not significantly different between the groups. Baseline levels of sCr and eGFR were similar (group A: 1.10±0.36 mg/dl and 73.4±21.8 mL·min–1·1.73 m–2, group B: 1.12±0.56 mg/dl and 75.7±23.9 mL·min–1·1.73 m–2; p=0.75 and p=0.15, respectively). With regard to procedural characteristics, the rate of primary or rescue PCI, treated vessel, extent of coronary disease and use of intra-aortic balloon pump did not differ between the groups. The amount of administered contrast medium and the VCCR in group A were slightly higher than in group B, although this difference was not statistically significant (227±102 vs. 214±96 ml, p=0.13 and 3.51 vs. 3.31, p=0.39). Notably, the two groups did not differ for MRS (5.85±4.98 vs. 5.94±4.96, p=0.82), corresponding to a predicted risk of CIN of about 14%21. A total of 68 (26%) patients in group B received a half dose of isotonic saline for overt heart failure.
PRIMARY AND SECONDARY ENDPOINTS
The primary combined endpoint of in-hospital death, need for dialysis and CIN was significantly lower in group A as compared to group B (9.2 vs. 18.7%, OR 0.43, 95% CI: 0.26-0.74, p=0.023) (Table 2) with a NNT of 11. Specifically, a significant reduction in the incidence of both in-hospital mortality (2.3 vs. 6.1%, OR 0.38, 95% CI: 0.14-0.97, p=0.049, NNT 27) and CIN (8.0 vs. 14.1%, OR 0.53, 95% CI: 0.30-0.93, p=0.03, NNT 17) was found, with no difference in the need for dialysis. In-hospital mortality was due to cardiogenic shock (n=3), left ventricular free wall rupture (n=1), complications following urgent coronary artery bypass grafting (n=1) or sudden death following probable stent thrombosis (n=1) in group A and cardiogenic shock (n=14), left ventricular free wall rupture (n=1), or sudden death following probable stent thrombosis (n=1) in group B.
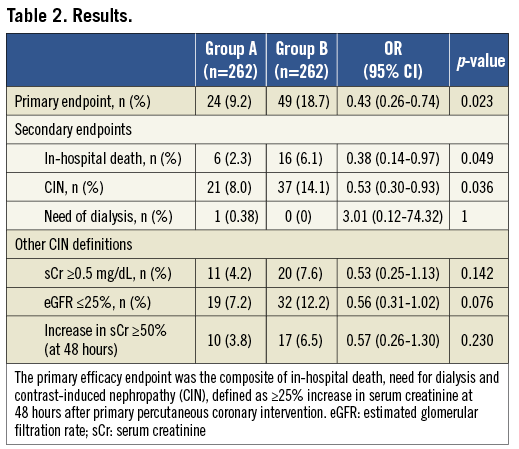
With regard to other definitions of CIN, both an increase ≥0.5 mg/dL of sCr (4.2 vs. 7.6%) or an increase ≥50% of sCr (3.8 vs. 6.5%) or a decrease of eGFR ≥25% (7.2 vs. 12.2%) tended to occur less frequently in group A than in group B, but this difference did not reach statistical significance (p=0.14, p =0.23 and p=0.08, respectively) (Table 2).
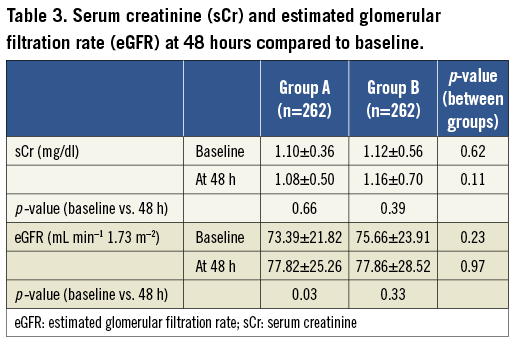
Moreover, at 48 hours after PCI, while no significant change in sCr was observed in either group (group A: from 1.10±0.36 to 1.08±0.50 mg/dl, p=0.66; group B: from 1.12±0.56 to 1.16±0.70, p=0.39), a significant increase in eGFR was observed in group A (from 73.39±21.82 to 77.82±25.26 mL·min–1·1.73 m–2, p=0.03) but not in group B (from 75.66±23.91 to 77.86±28.52 mL·min–1·1.73 m–2, p=0.33) (Table 3).
No difference in sCr at baseline and after PCI was observed in patients who died during hospitalisation in both groups (group A 2.07±1.07 at baseline and 2.64±1.05 at 48 hours, p=0.30 vs. 1.83±1.93 at baseline and 2.28±1.71 at 48 hours, p=0.22; p=0.40 for delta sCr between group A and group B).
SUBGROUP ANALYSIS
Subgroup analysis is shown in Figure 2. The primary endpoint was significantly reduced or tended to be reduced in group A compared to group B, irrespective of age, gender or diabetic status. Conversely, the benefit in group A appeared to be more evident in patients at high risk of CIN, such as patients with a baseline eGFR ≤60 mL·min–1·1.73 m–2 and a higher volume of administered contrast media, although the primary endpoint was significantly reduced in patients both at lower or at higher risk of CIN according to VCCR. Taking into consideration only patients in stages 2 (60-89 mL min–1 1.73 m–2) and 3 (3-59 mL min–1 1.73 m–2) of chronic kidney disease we found the occurrence of the primary endpoint in 16 patients in group A vs. 32 in group B (p=0.009). With regard to MRS, no significant difference in the distribution of patients between the two groups was present (Table 1). Of note, a lower incidence of the primary composite endpoint in medium-risk (1.9 vs. 7.6%, p=0.007; OR 0.18, 95% CI: 0.06-0.52) and in high-risk (0.8 vs. 3.4%, p=0.08; OR 0.20, 95% CI: 0.04-1.05) subgroups was observed, while no significant difference in low and very high-risk subgroups was present (4.2 vs. 5.3%, p=0.68 and 2.3 vs. 2.3%, p=1, respectively) (Figure 2). Finally, excluding patients presenting with shock at admission (18 patients in group A and 27 in group B), the incidence of the primary endpoint continued to be significantly lower in group A compared to group B (18 events [7.3%] in group A vs. 35 events [15%] in group B, p=0.013).
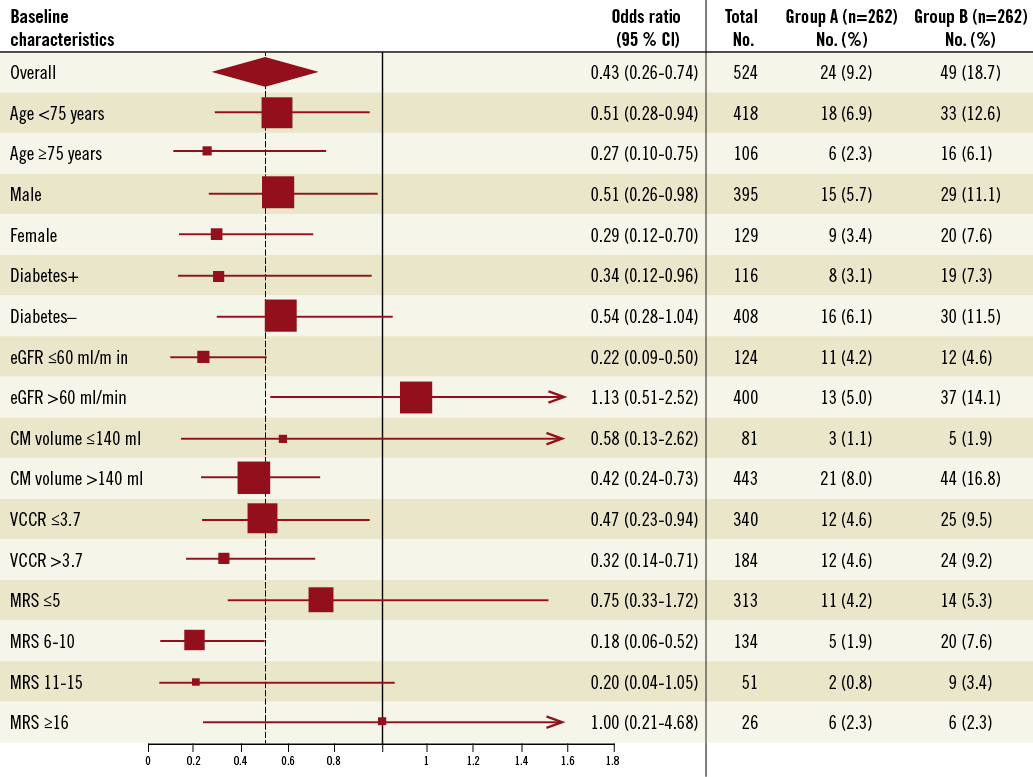
Figure 2. Rates of and odds ratios for the primary efficacy endpoint, overall and in various subgroups. The primary efficacy endpoint was the composite of in-hospital death, need for dialysis and contrast-induced nephropathy (≥25% increase in serum creatinine at 48 hours). The overall treatment effect is represented by the diamond, the left and right borders of which indicate the 95 per cent confidence interval. The dotted line represents the point estimate of the overall treatment effect. For subgroups, the size of each box is proportional to the number of patients in the individual analyses. The horizontal lines represent the 95 per cent confidence intervals. CM: contrast medium; eGFR: estimated glomerular filtration rate; MRS: Mehran risk score; VCCR: volume to creatinine clearance ratio
Discussion
The results of the BINARIO study consistently suggest that the combination of SB and high-dose NAC in a population of all-comers STEMI patients undergoing primary or rescue PCI is associated with a significant clinical benefit compared to saline hydration plus high-dose NAC. Of note, the reduction in the rate of primary endpoint was driven by a significant beneficial effect on both in-hospital death and CIN. This benefit was particularly evident in patients at medium to high risk of CIN according to MRS.
BENEFIT OF HYDRATION WITH SODIUM BICARBONATE AND NAC
CIN represents a serious complication of coronary interventional procedures, often being associated with prolonged hospitalisation, increased costs and higher mortality. In the setting of STEMI undergoing urgent PCI, the incidence of CIN is much higher than in elective procedures, with a reported incidence ranging from 10 to 30%8,12,18.
Several different pharmacological (using atrial natriuretic peptide, fenoldopam, dopamine, etc.), mechanical (by removal of contrast medium from the coronary sinus) or combined (such as the RenalGuard System™; PLC Medical Systems, Inc., Milford, MA, USA) approaches were developed to limit CIN occurrence19,20. Nevertheless, despite the use of these complex and expensive techniques, the rate of CIN continues to be high and its occurrence largely unpredictable. This could be due to the pathophysiology of CIN which is rather complex and not yet completely understood. Direct toxicity of contrast medium, which is a function of time and concentration of iodinated contrast in the renal tubules and collecting ducts, can generally be counteracted by preprocedural hyperhydration, as commonly suggested by international guidelines21. Unfortunately, in the setting of STEMI, prehydration before primary PCI is not feasible. Free radical generation represents another potential target of nephroprotective strategies. Although data on the protective effect of NAC in the elective setting are conflicting and have recently been challenged by the large Acetylcysteine for Contrast-Induced Nephropathy Trial (ACT)22,23, in primary PCI Marenzi et al demonstrated that high-dose NAC is associated with net clinical benefit12.
SB exerts its nephroprotective effect through a free radical scavenging mechanism mediated by renal tubular fluid alkalinisation24,25. Overall, the results of SB in the elective setting are conflicting, suggesting at best a mild nephroprotection in comparison to sodium chloride in patients with moderate to severe renal insufficiency26-29. Moreover, very recently a large trial failed to demonstrate a beneficial effect of intravenous SB (without concomitant NAC) in patients with renal insufficiency undergoing elective intravascular procedure compared to a standard hydration with volume supplementation with either sodium chloride or SB mainly administered orally30. Nevertheless, the combination of SB and NAC has been shown possibly to protect against CIN. In particular, the REMEDIAL trial demonstrated that a strategy combining SB and NAC significantly reduced CIN in a high-risk population undergoing elective procedures as compared to NAC plus placebo or NAC plus ascorbic acid13.
In the BINARIO study we prospectively tested the efficacy of an SB-based hydration, according to the REMEDIAL protocol13 plus high-dose NAC in a population of all-comers STEMI patients undergoing primary or rescue PCI. The control group was represented by an historical cohort of retrospectively-enrolled STEMI patients treated by saline infusion and high-dose NAC, according to our previous internal guidelines based on the protocol of Marenzi et al12. SB-based hydration yielded a significant reduction in the combined primary endpoint of in-hospital death, need for dialysis and CIN. The results of the study suggest that the combination of SB and NAC might exert a synergistic protective effect, possibly mediated by a decrease in reactive oxidised species (ROS) generation and scavenging. This might be particularly important considering that ROS generation is amplified in the setting of acute myocardial infarction31. In this regard, it is worth noting that the beneficial effect of SB was evident despite the fact that patients treated with saline were hydrated with a larger volume supplementation (an almost three-times larger volume), thus allowing us to hypothesise a primary role for ROS blockade and urine alkalinisation over volume supplementation in the prevention of CIN in the setting of STEMI.
EFFECT OF SODIUM BICARBONATE AND NAC ON CIN AND MORTALITY
The Reno-Protective Effect of Hydration With Sodium Bicarbonate Plus N-acetylcysteine in Patients Undergoing Emergency Percutaneous Coronary Intervention (RENO) trial has previously tested the efficacy of the combination of SB and NAC in urgent percutaneous coronary procedures, reporting a remarkable reduction of the rate of CIN compared to saline hydration and NAC in the setting of emergency PCI18. Although the differences between the two studies make them not fully comparable, especially in terms of the proportion of STEMI patients enrolled, different doses and administration strategies, and amount of contrast volume, our data are in keeping with a consistent reduction in the rate of CIN with the combination of SB and NAC. It must be noted that the rate of CIN in our control cohort (14%) was much lower than the one observed in the control group of the RENO study (30%), but higher than that of the subgroup of the Marenzi study which received a scheme of NAC administration (8%)12 identical to ours. Again, these differences can be partly explained by the characteristics of the study patients, but it is worth noting that the observed incidence of CIN in our control cohort coincides with that predicted according to mean MRS (5.94±4.95)17. More recently, in a similar clinical setting of STEMI patients undergoing primary PCI, Maioli et al demonstrated that strategies of volume expansion, both by preprocedural SB and by postprocedural standard saline, reduce the rate of CIN (calculated as ≥25% increase in sCr) without affecting the incidence of clinical endpoints, such as death or myocardial infarction32. This could suggest that the reduction in the rate of CIN may be due to a dilution of sCr levels for hyperhydration more than to a real nephroprotection. This hypothesis could be confirmed by the slow rise in the course of sCr levels which exactly parallels the control group33. On the other hand, it is worth noting that in the BINARIO study, in a more complex population, SB-based hydration was also associated with a significant decrease of in-hospital mortality. Despite the absence of significant differences in baseline clinical, angiographic or procedural characteristics between the two groups, since the study did not aim to demonstrate a significant reduction in mortality, we cannot exclude that this might simply be due to chance. That said, it must be highlighted that even in the study of Marenzi et al a strategy of nephroprotection in the setting of STEMI was associated with a significant reduction in mortality12. Thus, our finding is only partly surprising and is in line with the concept that CIN is a strong predictor of a poor outcome, along with the hypothesis that antioxidant therapy might also have an extra-renal protective effect. Accordingly, both clinical and experimental studies in acute myocardial infarction showed a reduction in infarct size and an improvement in left ventricular function associated with administration of antioxidants and free radical scavengers34.
SUBGROUP ANALYSIS
The benefit conferred by SB and NAC was present among the majority of subgroups, thus confirming the robustness of the effect. Subgroup analysis according to MRS appears of particular interest. MRS is a simple clinical tool for risk assessment of CIN, and has recently been shown to be an independent predictor of mortality35. The analysis of the incidence of the primary endpoint in the four subgroups according to MRS suggests a significant benefit of an SB-based strategy in the intermediate categories only. It can be speculated that, while the subgroup with MRS score ≤5 might reflect a category of patients at too low risk of in-hospital events to demonstrate clearly a statistically significant beneficial effect of SB-based hydration, in patients at very high risk (MRS score ≥16) the beneficial effect of the SB hydration might be insufficient. For these patients it is plausible that the increase in sCr might more probably reflect an acute kidney injury secondary to haemodynamic instability rather than a pure contrast medium toxic action. Unlike the results observed in these subgroups, the benefit of SB was clearly evident in patients at medium risk (MRS score 6-10) and at high risk (MRS score 11-15). However, given the practical impossibility of calculating MRS accurately at the time of urgent PCI and, more importantly, the very favourable cost-effectiveness and safety profile of the combination of SB and NAC (only 11 patients are needed to treat to prevent the occurrence of one event), we believe that this strategy could reasonably be proposed for all patients with STEMI undergoing urgent PCI. In addition, the notion that patient clinical risk profile might be more important than procedural variables in determining the benefit of SB and NAC is further confirmed by the analysis of subgroups according to baseline eGFR. Indeed, the nephroprotective effect of SB was concentrated in the subgroup of patients with mildly or moderately impaired renal function and only a marginal benefit was observed in patients with preserved baseline renal function.
STUDY LIMITATIONS
Although the endpoints of the study were prospectively identified and the study design was previously published (Clinical trials.gov, NCT01218178), despite the absence of difference in demographic, clinical and procedural characteristics of the two study groups, the main limitation of the study concerns the registry design that involves a retrospectively-enrolled cohort. This limitation implies that our results should be confirmed in a prospective, randomised double-blind clinical trial. However, considering that SB plus NAC is a very low-cost, safe and possibly effective nephroprotection strategy, we feel that a widespread SB-based hydration could yet be proposed as a standard approach in STEMI patients undergoing primary PCI.
According to current STEMI guidelines most of the patients received potentially nephroprotective drugs, such as ACE inhibitors or ARBs. Although we cannot exclude a possible influence of these therapies on renal function, the absence of a significant difference in the prevalence of their use between group A and group B makes a potential confounding effect on the main results very unlikely.
About 85% of patients received a large volume of contrast dye (>140 ml), underscoring that primary or rescue PCI is at high risk of CIN. Nevertheless, the inclusion of patients in whom a small amount of contrast media was used could have diluted the favourable effect of SB in comparison to isotonic saline.
In the present study we calculated eGFR using the MDRD formula which offers a rough estimation of renal function. Despite the existence of other methods which are intended to calculate eGFR more accurately, especially when sCr fluctuations are possible, the MDRD formula continues to be the most frequently used equation in studies on CIN and we chose it for consistency. We avoided calculating creatinine clearance from collection of urine output, because this method, which is considered the gold standard, is not easily feasible in a large population study. For these reasons, we and others in similar studies decided to use as the primary endpoint not a modification in eGFR but the incidence of CIN, defined as ≥25% relative increase in sCr.
Conclusions
Our data, obtained in a real world large population of retrospectively and prospectively enrolled STEMI patients undergoing primary or rescue PCI, suggest that the strategy of volume supplementation by SB-based hydration in addition to high-dose NAC is associated with a net clinical benefit, including a decrease in CIN occurrence and in-hospital mortality. The limitation of having included a retrospectively enrolled control cohort makes the BINARIO a hypothesis-generating study. Nevertheless, the observed high clinical benefits of this extremely low-cost form of treatment highlight the urgent need for controlled randomised trials in large patient populations.
Acknowledgements
A.M. Leone is personally indebted to the nursing staff of the Intensive Cardiac Care Unit without whose help this study could not have been conducted. The study was supported by the Catholic University of the Sacred Heart and by the Policlinico Casilino/ASL RMB. The study received no funding from manufacturers of contrast agents or suppliers of saline or bicarbonate. A.M. Leone and A.R. De Caterina had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix
PERCUTANEOUS CORONARY INTERVENTION
All STEMI patients were pretreated with aspirin (500 mg) and clopidogrel loading dose (600 mg). The radial approach was attempted as first choice unless the femoral route was clinically indicated. A bolus of 5,000 UI of unfractionated heparin was directly administered in the catheterisation laboratory, followed by additional intraprocedural boluses to maintain the activated clotting time of 250-350 seconds (or 200-250 seconds when abciximab was used). Abciximab administration was advised (0.25 mg/kg intracoronary bolus plus i.v. infusion of 0.125 g/kg/min for 12 h), but left to the operator’s discretion. The strategy of treating the infarct-related artery only was adopted in all patients. After crossing the target lesion with the guidewire, manual thrombus aspiration followed by direct stent implantation (either bare metal or drug-eluting stent) was attempted if judged possible by the operator, whereas in the remaining cases predilation with an undersized balloon was used before stent implantation. All decisions regarding procedural details, including contrast doses, vasoactive drugs administration or intra-aortic balloon pump implantation were left to the discretion of the interventional cardiologist. Notably, no major technical changes in PCI procedures were introduced in the whole period of enrolment. Low-osmolality nonionic contrast media iomeprol and iopamidol (Iomeron 350 and Iopamiro 370; Bracco S.p.a, Milan, Italy) with 350-370 mg/ml of iodine content was used in all cases.
DEFINITIONS OF CARDIOVASCULAR RISK FACTORS
Medical history including cardiovascular risk factors, full anthropometric assessment and routine laboratory tests were obtained from all patients. Cardiovascular risk factors: age, gender, diabetes defined according to American Diabetes Association criteria1, dyslipidaemia defined according to National Cholesterol Education Program screening criteria2, hypertension defined according to Joint National Committee 7 criteria3, cigarette smoking (>1 cigarette/day) and family history of early acute coronary syndromes (documented acute coronary syndrome before 60 years of age in at least one first-degree relative).
Online references

