Abstract
Aims: Worsening renal function in patients undergoing percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI) is associated with adverse clinical outcomes. We hypothesised that platelet glycoprotein IIb/IIIa receptor inhibitors (GPI) may decrease the rate of renal function deterioration in these patients through attenuation of platelet aggregation and the possible improvement of renal rheology and haemodynamics.
Methods and results: Based on prospectively collected data, we analysed rates of renal function deterioration in 603 consecutive patients (mean age 58±13 years, males 82%) with STEMI treated with primary or rescue PCI. Renal function deterioration was defined as an increase in serum creatinine level of ≥25% and/or ≥0.5 mg/dl at any time point post-PCI during index hospitalisation compared with baseline value. Outcomes were stratified by treatment with GPI. Patients treated with GPI (n=442) vs. patients who were not treated with GPI (n=161) had significantly lower rates of serum creatinine increase of ≥25% compared with baseline (22.9% vs. 31.9%, P=0.02, respectively), of serum creatinine increase ≥0.5 g/dL (4.1% vs. 8.8%, P=0.02). Treatment with GPI was associated with significantly lower mean maximal increase in serum creatinine level compared with baseline value (0.14±0.38 vs. 0.25±0.45 mg/dL, P=0.005). Rates of major bleeding did not differ significantly between the two groups (7.3% vs. 5.9%; P=0.42), while 30-day mortality was significantly lower in patients treated with GPI (2.3% vs. 7.5%; P=0.005). By multivariable analysis, treatment with GPI was an independent predictor of freedom from renal function deterioration (odds ratio 0.53; 95% confidence interval 0.33-0.86; P=0.01).
Conclusions: In this analysis, administration of GPI to patients with STEMI treated with primary PCI was associated with lower rates of worsening renal function and lower 30-day mortality.
Introduction
Renal function deterioration after percutaneous coronary intervention (PCI) is an independent predictor of morbidity and mortality in patients with ST-segment elevation myocardial infarction (STEMI)1-3. Though the exact mechanisms of renal function decline after PCI are not known, several pathways have been implicated including ischaemic injury, direct cytotoxicity on renal structures, oxidative stress, apoptosis, and haemodynamic changes leading to impairment of renal blood flow4-9. Platelet glycoprotein IIb/IIIa receptor inhibitors (GPI) are potent suppressors of platelet aggregation and have been shown to decrease rates of ischaemic complications and to improve survival in patients with STEMI treated with primary PCI10-14. We hypothesised that GPI might improve renal function by attenuation of platelet aggregation and improving renal perfusion. We therefore assessed impact of GPI on renal function and clinical outcomes in consecutive patients with STEMI treated with primary PCI.
Methods
All patients undergoing primary or rescue PCI at a high-volume tertiary referral centre from January 2000 through December 2006 were included in this analysis. Per standard practice, GPI were not administered to patients with known thrombocytopenia (platelet count <100,000 per cubic millimetre), haemorrhagic diathesis, uncontrolled hypertension, a history of haemorrhagic stroke, and a history of major trauma, major surgery, active bleeding, or ischaemic stroke within two months prior to procedure. In patients without contraindications, treatment with GPI was at the discretion of the operators. Patients requiring dialysis and patients with known severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2 were excluded from the study. Data were prospectively entered in a database that contained demographic, clinical, and angiographic information. All adverse events were source-documented. The study was conducted and approved by the Institutional Review Board of the hospital, and all patients signed written informed consent.
Prior to catheterisation, patients received 200-325 mg of aspirin and clopidogrel 300 mg. Unfractionated heparin was administered during the procedure in a dose sufficient to maintain an activated clotting time of 250-300 seconds. Bivalirudin was administered as an intravenous bolus of 0.5 mg/kg followed by the infusion 1.75 mg/kg/h. Dosages of all GPI were as per the package insert and were adjusted for renal impairment. Laboratory data, including values of haemoglobin and serum creatinine, were collected on arrival and every 24 hours for at least three days.
Endpoints and definitions
The primary endpoint, renal function deterioration, was defined as an increase in serum creatinine level of ≥25% and/or ≥0.5 mg/dl at any time point post-PCI during index hospitalisation compared with baseline value. Major bleeding was defined as fatal or disabling bleeding, bleeding resulting in transfusion and/or prolongation of hospitalisation. Transfusion of blood product was performed at the investigators’ discretion for clinical indications. Baseline estimated glomerular filtration rate (eGFR) was calculated using Levey modified MDRD formula15.
Statistical analysis
Continuous variables are expressed as mean±1 standard deviation and compared using 2-sample t-tests. Categorical data are presented as frequencies and compared using chi-square statistics or Fisher’s exact test. All tests were 2-sided. Analysis of predictors of renal function deterioration was performed using multivariable logistic regression. The following variables were forced into the model: age, gender, diabetes mellitus, hypertension, current smoking, heart rate on admission, systolic and diastolic blood pressure on admission, anterior location of MI, Killip class at admission, volume of contrast medium, and administration of GPI. A 2-sided 95% confidence interval (CI) was constructed around each point estimate of odds ratio (OR). Model calibration was assessed by the Hosmer-Lemeshow goodness-of-fit test. The linearity of the logit among the continuous variables used in the multivariable analysis was confirmed with the Box–Tidwell test. All statistical analyses were performed using the SPSS statistical software (Version 15.0).
Results
Patient population
A total of 603 patients with STEMI underwent primary or rescue PCI during the study period. Treatment with GPI was administered to 402 (66.7%) patients. Among patients who were administered a GPI, the majority were treated with eptifibatide (86%) followed by tirofiban (8%) and abciximab (6%).
Baseline characteristics of the patients stratified as a function of GPI treatment are presented in Table 1. Patients treated with GPI tended to be younger and more commonly males, had significantly lower prevalence of diabetes mellitus and Killip class ≥2 on admission, and had better baseline renal function as assessed by baseline eGFR. The proportion of patients with anterior location of MI and the value of mean left ventricle ejection fraction did not differ significantly between the two groups. The volume of contrast media administered during the procedure was also similar between patients who were treated with GPI vs. those who were not (191±66 ml vs. 200±64 ml, respectively, P=0.64).
Renal function post-primary PCI
The primary endpoint, renal function deterioration, defined as an increase in baseline serum creatinine of ≥25% and/or ≥0.5 mg/dl at 24 to 72 hours post-PCI compared with baseline value occurred significantly less frequently in patients who were administered GPI compared to those who were not (22.9% and 31.7%, respectively, P=0.024). By univariate analysis (Table 2), renal function deterioration post-index PCI had significant association with older age, non-smoking status, anterior MI, Killip class ≥2, lower baseline eGFR and treatment with GPI. Neither diabetes nor volume of contrast agent predicted renal function deterioration.
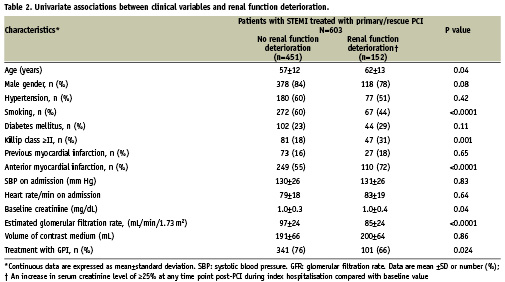
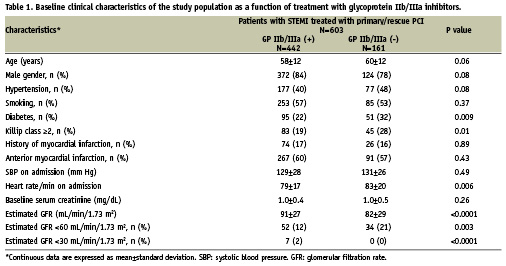
The proportion of patients with an increase in serum creatinine level of ≥25% at any time point post-PCI during index hospitalisation compared with baseline value (Figure 1) was also significantly lower among patients who were administered GPI compared with patients who were not (22.9% vs. 31.9%, P=0.02). The same was true with respect to the proportion of patients with an increase in serum creatinine level of ≥0.5 mg/dL (4.1% vs. 8.8%, P=0.02). Finally, patients treated with GPI had significantly lower mean maximal increase in serum creatinine level compared with baseline value (0.14±0.38 vs. 0.25±0.45 mg/dL, P=0.005).
Figure 1. Univariate analysis: renal function deterioration and treatment with GPI.
Other clinical outcomes
Thirty-day mortality was significantly lower in patients treated with GPI compared to those who were not (2.3% vs. 7.5%, respectively; P=0.005) (Figure 2) Rates of major bleeding did not differ significantly between the two groups (7.3% vs. 5.9%, respectively; P=0.42). Mean haemoglobin change between baseline and nadir values during the index hospitalisation was close between the two groups (1.6 g/dL±1.3 vs. 1.5±1.3 g/dL, P=0.26).
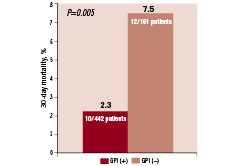
Figure 2. Thirty-day mortality stratified by treatment with GPI.
Multivariable analysis
In a multivariable logistic regression model, administration of a GPI during PCI had a protective influence on renal function (OR 0.53; 95% confidence interval [CI], 0.33-0.86; P=0.01). Other independent predictors of renal function deterioration included older age (per 10 years increase) (odds ratio [OR]=1.47; 95% CI, 1.21–1.89; P<0.0001), anterior MI (OR 2.20; 95% CI 1.37-3.55; P=0.001), Killip class ≥2 (OR 2.0; 95% CI 1.19-3.40; P=0.009), and lower baseline eGFR (OR 1.31; 95% CI 1.20-1.43).
Discussion
The main findings of the present analysis, the first to our knowledge to assess the impact of glycoprotein IIb/IIIa inhibition on renal function in consecutive patients undergoing primary/rescue PCI for STEMI, are as follows:
1) Treatment with GPI was associated with lower risk of renal function deterioration post PCI;
2) Positive impact of GPI on renal function was independent of other covariates;
3) Patients treated with GPI had lower rates of 30-day mortality.
In this retrospective analysis based on a single-centre experience, decline in renal function occurred in 25.2% of the patients treated with primary or rescue PCI for STEMI. Renal function deterioration was independently predicted by well recognised factors including older age and lower eGFR16-18. There was a 30% increase in odds of renal function deterioration per each 10 units decrease in eGFR. Anterior myocardial infarction was also associated with higher rates of renal function deterioration post-PCI. This is likely due to a larger infarct area and subsequently worse left ventricular performance in patients with anterior vs. non-anterior infarctions compatible with more prominent haemodynamic changes and worse renal function19.
Neither diabetes nor volume of contrast medium predicted renal function deterioration in this analysis. Although the presence of diabetes mellitus is a recognised risk factor for the development of contrast-induced nephropathy, in diabetics with preserved renal function and in the absence of other risk factors, the rates of contrast-induced nephropathy are usually comparable to those of a healthy population20, while clinically important renal function deterioration typically occurs only in a subset of diabetics with underlying renal insufficiency3,21. The correlation between the amount of contrast media and the risk of contrast-induced nephropathy has been well documented17,22. According to McCullough et al2, the risk of contrast-induced nephropathy is minimal in patients receiving <100 ml of contrast2. However, according to different sources, the relatively safe cut-off point of contrast amount may be as high as 220 ml22-24. The mean volume of contrast media was relatively low in this study that might be a possible explanation for the lack of relationship between contrast volume and rates of renal function deterioration. In the randomised Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial, the median amount of contrast administered was approximately 300 ml18.
The finding of lower rates of renal function deterioration in relation to the use of GPI has not been previously reported. Several possible mechanisms may explain the association with the use of GPI and the lesser degree of renal function decline in patients with STEMI treated with primary PCI. GPI may have a positive impact on renal blood flow that is known to be compromised by contrast media. Several contrast media including low-osmolar and iso-osmolar agents have been demonstrated to decrease the cortical blood flow26,27. In addition, exposure to both ionic and non-ionic contrast media has been shown to result in an increase in plasma viscosity, erythrocyte aggregation, and platelet reactivity index8,28. GPI may improve renal perfusion by reducing platelet aggregation. Furthermore, by improving flow in epicardial vessels and in microcirculatory bed, GPI may improve central haemodynamics resulting in the enhancement of renal perfusion29. Higher rates of Thrombolysis in Myocardial Infarction flow grade 3 and lower rates of procedural complications known to be associated with the use of GPI may simplify PCI resulting in the lower amount of contrast media11.
In this analysis, 30-day mortality was lower in patients treated with GPI compared with those who were not. The data regarding the relationship between GPI and mortality in patients treated with primary PCI is conflicting. In the randomised CADILLAC trial, 30-day mortality was not related to administration of abciximab (2.5% in PTCA arm vs. 1.1% PTCA plus abciximab arm vs. 2.2% in stent arm and 2.7% in stent plus abciximab arm, P=0.31). However, in the randomised Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up (ADMIRAL) trial, rates of 30-day mortality were remarkably lower in patients treated with abciximab compared with placebo though not reaching statistical significance (3.4% vs. 6.6%, P=0.19)10. Moreover, a significant reduction in 30-day mortality was observed with abciximab in a meta-analysis of 11 angioplasty trials (2.4% vs. 3.4%; P=0.047)14. In another meta-analysis, assessing impact of abciximab (eight trials), eptifibatide (three trials) or tirofiban (one trial) versus placebo, there was survival benefit in favour of GPI as class translating into prevention of approximately one of every three deaths within 30 days post PCI13.
Limitations
This was a non-randomised, single-centre study subject to spatial bias. However, in this analysis, the proportion of patients who received a GPI during the first half of the study period as opposed to the second half of the study period when robust evidence on the safety and efficacy of GPI in the management of patients with STEMI undergoing primary PCI became available, was close10,14. Despite the fact that the data were collected prospectively, this post hoc study was not pre-specified and should thus be considered hypothesis generating. Our analysis was not powered to determine differences in clinical outcomes in relation to use of GPI; therefore, obtained data may be due to chance alone (type I error). Although multivariable statistical modelling was used to correct for baseline and procedural differences, other unmeasured variables may not have been controlled for. Given the relatively small number of patients treated with tirofiban, the effect of GPI as a drug class on outcomes has been assessed. Nonetheless, this analysis represents the first report on the impact of GPI on renal function in patients with STEMI treated with primary PCI. Prospective randomised study is warranted to clarify whether GPI are beneficial in diminishing renal function deterioration in patients undergoing contrast media exposure in the setting of STEMI.
Conclusions
In this series of consecutive patients undergoing primary or rescue PCI for STEMI, administration of GPI was independently associated with lower rates of renal function deterioration and lower in-hospital mortality. Given the adverse prognostic implications of renal function deterioration after PCI on the prognosis of patients with STEMI, the impact of GPI on renal function should be further elucidated in a randomised study.
Acknowledgements
The authors express their appreciation to Dina Azulay for her outstanding secretarial assistance.

