Abstract
Aims: In recent years, intracoronary bolus abciximab has emerged as an alternative to the standard intravenous route in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI). The aim of the current study was to perform an individual patient-level pooled analysis of randomised trials, comparing intracoronary versus intravenous abciximab bolus use in STEMI patients undergoing primary PCI.
Methods and results: Individual data of 3,158 patients enrolled in five trials were analysed. Reperfusion endpoints were: post-procedural Thrombolysis in Myocardial Infarction (TIMI) 3 flow, myocardial blush grade (MBG) 2/3 and complete ST-segment resolution. The primary clinical endpoint of interest was the composite of death and reinfarction at 30 days. Compared with the intravenous route, intracoronary abciximab bolus administration did not improve TIMI 3 flow (odds ratio [OR] 1.19; 95% confidence interval [CI]: 0.90-1.59; p=0.23) and complete ST-segment resolution (OR 1.22, 95% CI: 0.92-1.63, p=0.17), but increased MBG 2/3 occurrence (OR 1.83, 95% CI: 1.05-3.18, p=0.03). At 30-day follow-up, intracoronary bolus abciximab did not reduce the risk of death and reinfarction (OR 0.78, 95% CI: 0.55-1.10, p=0.16), death (OR 0.77, 95% CI: 0.51-1.17, p=0.22), reinfarction (OR 0.79, 95% CI: 0.46-1.33, p=0.38) and stent thrombosis (OR 0.77, 95% CI: 0.43-1.35, p=0.36) as compared with intravenous administration.
Conclusions: In STEMI patients undergoing primary PCI, intracoronary abciximab does not provide additional benefits as compared with standard intravenous treatment and, therefore, it should not be recommended as the default route of administration in this setting.
Introduction
Primary percutaneous coronary intervention (PCI) represents the preferred reperfusion strategy in patients with ST-elevation myocardial infarction (STEMI), because it is more effective than fibrinolytic agents in reducing cardiovascular events, including death1. Despite successful infarct-related coronary artery flow restoration, impaired myocardial reperfusion is frequently observed, resulting in a higher infarct extent, reduced ventricular function recovery and increased mortality2,3.
Potent antiplatelet inhibition with glycoprotein IIb/IIIa receptor inhibitors represents a well-established adjunctive therapy during primary PCI, aiming to improve coronary microcirculation. Several randomised trials showed decreased early and late mortality rates in STEMI patients with intravenous administration of abciximab, a chimeric monoclonal antibody fragment inhibiting the platelet-bound glycoprotein IIb/IIIa receptor4,5. Experimental studies suggested additional antiplatelet, antithrombotic and anti-inflammatory properties when a higher local drug concentration is achieved through intracoronary abciximab bolus administration6,7. Despite the fact that AHA/ACC guidelines provided a Class IIb recommendation for intracoronary abciximab administration in patients with STEMI undergoing primary PCI8, randomised trials showed mixed results for a variety of surrogate and combined clinical endpoints9-11.
Therefore, to evaluate the impact of different abciximab route strategies on reperfusion and clinical outcomes, we planned an individual patient-level pooled analysis of randomised trials, comparing intracoronary with intravenous abciximab bolus administration in STEMI patients undergoing primary PCI.
Methods
PATIENT POPULATION
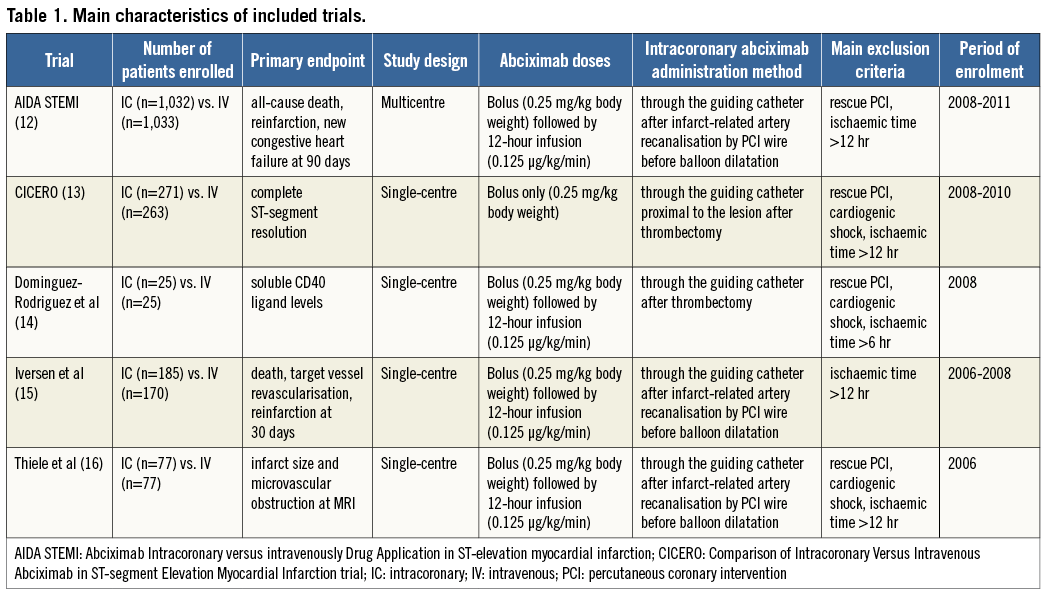
We performed a patient-pooled analysis of five randomised clinical trials comparing the intracoronary with the intravenous bolus administration of abciximab in patients undergoing primary PCI12-16. A summary of the principal trial characteristics is reported in Table 1.
All principal investigators provided individual patient data by using an electronic dataset. All data were checked for completeness and consistency and compared with the results of original publications.
TRIAL QUALITY ASSESSMENT
The risk of bias was evaluated in accordance with the Cochrane Collaboration methods17 and considering the following methodological items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete data outcome, selective reporting, other bias and sample size calculation.
ENDPOINTS AND DEFINITIONS
REPERFUSION ENDPOINTS
Post-procedural Thrombolysis in Myocardial Infarction (TIMI) 3 flow, myocardial blush grade (MBG) 2/3 and complete (>70%) ST-segment resolution at 60-90 min were assessed as reperfusion endpoints.
CLINICAL ENDPOINTS
The primary clinical endpoint was the composite of death and reinfarction. Secondary endpoints were the individual endpoints of death, reinfarction and stent thrombosis, according to Academic Research Consortium criteria18. All clinical endpoints were evaluated at 30-day follow-up and managed according to the intention-to-treat principle.
STATISTICAL ANALYSIS
Statistical analyses were obtained by using Review Manager 5.2 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) and IBM SPSS 20.0 (IBM, Armonk, NY, USA). Odds ratio (OR) and 95% confidence intervals (95% CI) were used as summary statistics. For study-level analysis, the pooled OR was calculated by using the fixed effect Mantel-Hænszel model, while in the case of significant heterogeneity across studies the random effects DerSimonian and Laird model was used instead. The Breslow-Day chi-squared test was calculated to test the statistical evidence of heterogeneity across the studies (p<0.1). In addition, we used the I2 statistic, which describes the percentage variation across studies that is due to heterogeneity rather than chance19.
Survival analyses were performed using the Mantel-Cox method stratified by the trial. Survival curves are presented as simple, non-stratified Kaplan-Meier curves across all trials. Exploratory multivariable analyses were done in order to assess predictors of reperfusion indices and primary clinical endpoints by a binary stepwise logistic regression analysis. The final model included variables significantly associated at univariate analysis. Statistical significance was assumed for p<0.05.
SENSITIVITY ANALYSES
In order to confirm the results from the traditional meta-analysis, Bayesian random effects meta-analyses were performed using WinBUGS software (version 1.4; MRC Biostatistics Unit, Cambridge, UK). Details and the code used for the analysis are reported in the Online Appendix. The influence of single studies on the summary estimates was examined graphically by checking how the elimination of each study affected the resulting summary estimate of OR.
Results
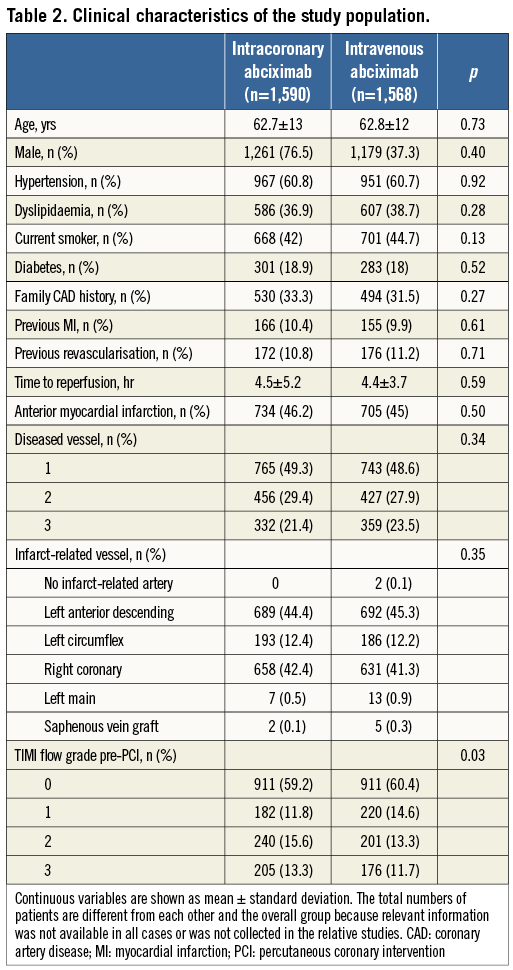
A total of 3,158 patients were included in this pooled analysis (1,590 or 50.43% randomly assigned to intracoronary abciximab and 1,568 or 49.7% randomly assigned to intravenous abciximab). No significant heterogeneity across trials was observed in the analysis of reperfusion and clinical endpoints. All five trials were assessed as a low to moderate risk for bias. Periprocedural anticoagulation consisted of intravenous unfractioned heparin, and abciximab bolus was administered through the guiding catheter in all patients randomised to the intracoronary route (Table 1). Baseline characteristics of the pooled population are reported in Table 2.
REPERFUSION ENDPOINTS
POST-PROCEDURAL TIMI FLOW
Data were available for 3,059 patients. As reported in Figure 1A, intracoronary abciximab did not increase the proportion of patients achieving post-procedural TIMI 3 flow as compared with intravenous abciximab (87.5% vs. 86.2%, respectively, OR 1.19, 95% CI: 0.90 to 1.59, p=0.23).
MYOCARDIAL BLUSH
Data were available for 730 patients. As reported in Figure 1B, intracoronary abciximab increased MBG 2/3 rates as compared with intravenous administration (80.7% vs. 71.8%, respectively, OR 1.83, 95% CI: 1.05 to 3.18, p=0.03).
ST-SEGMENT RESOLUTION
Data were available for 2,331 patients. As reported in Figure 1C, intracoronary compared with intravenous abciximab did not increase the proportion of patients with complete ST-segment resolution after PCI (42.6% vs. 38.8%, respectively, OR 1.22, 95% CI: 0.92 to 1.63, p=0.17).
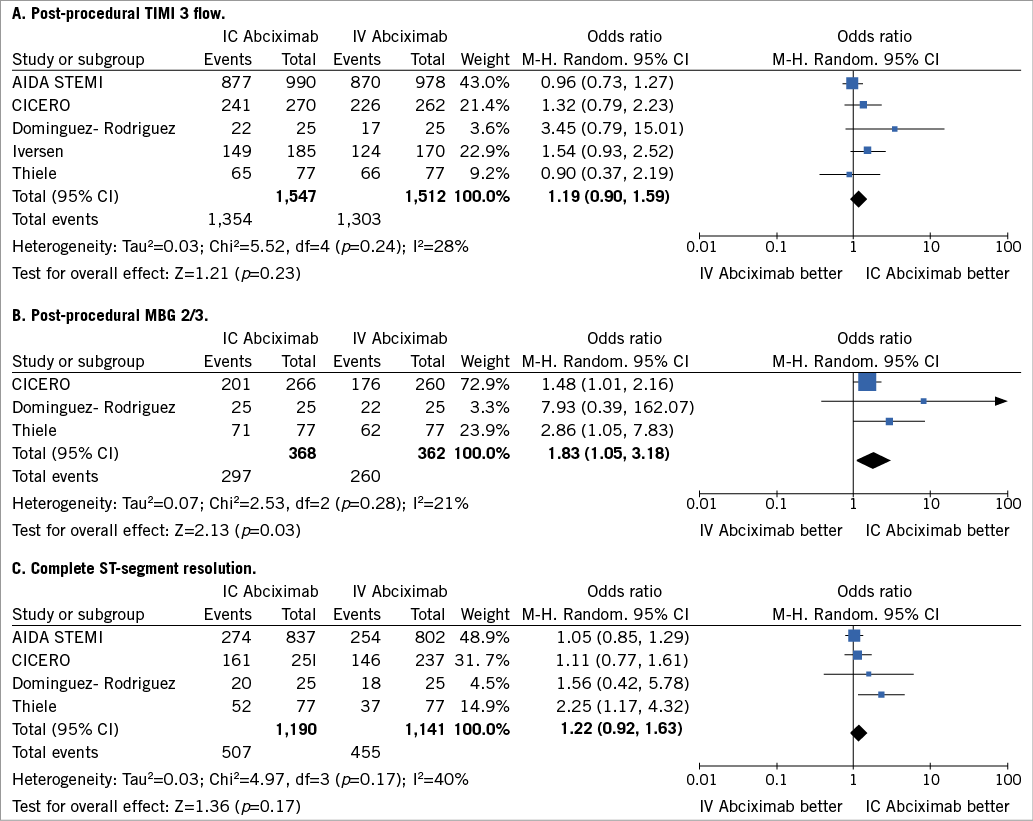
Figure 1. Analysis of reperfusion endpoints according to bolus abciximab route. (A) Post-procedural TIMI 3 flow; (B) post-procedural MBG 2/3; (C) complete ST-segment resolution. The squares and the horizontal lines indicate the odds ratio (OR) and the 95% CI for each trial; the size of each square is proportional to the statistical weight of a trial in the meta-analysis; diamond indicates the effect estimate derived from meta-analysis, with the centre indicating the point estimate and the left and the right ends the 95% CI. AIDA STEMI: Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction; CICERO: Comparison of Intracoronary Versus Intravenous Abciximab in ST-segment Elevation Myocardial Infarction trial; M-H: Mantel-Hænszel model; IC: intracoronary; IV: intravenous; MBG: myocardial blush grade; TIMI: Thrombolysis In Myocardial Infarction
CLINICAL ENDPOINTS
At 30-day follow-up, the composite of death and reinfarction occurred in a total of 135 patients (4.3%). As reported in Figure 2, intracoronary abciximab did not improve the composite of death or reinfarction as compared with intravenous abciximab (3.8% vs. 4.8%, respectively, OR 0.78, 95% CI: 0.55 to 1.10, p=0.16). There were no substantive differences among subgroups (Figure 3), with the exception of diabetic patients in whom intracoronary abciximab significantly lowered the risk of death or reinfarction (OR 0.50, 95% CI: 0.26 to 0.99, p=0.046, p-interaction=0.13).
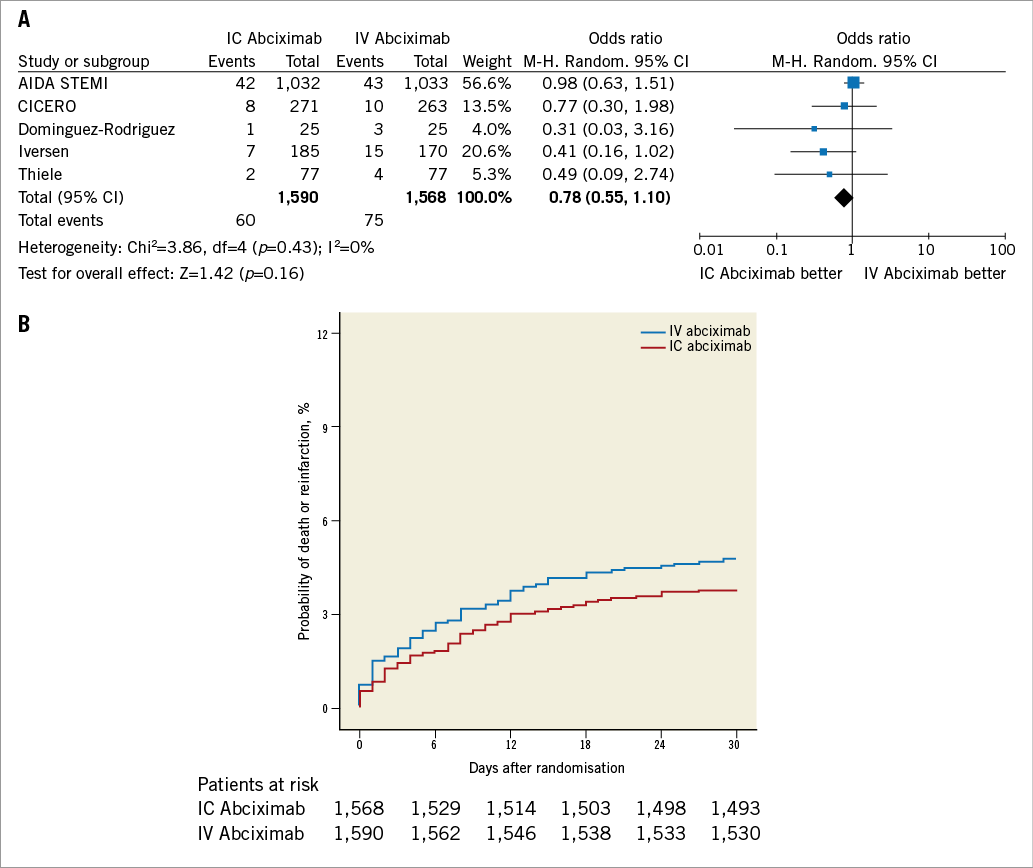
Figure 2. Analysis of mortality and reinfarction according to bolus abciximab route. A) Odds ratio (OR) of mortality and reinfarction associated with intracoronary versus intravenous bolus abciximab administration for individual trials and pooled population. B) Kaplan-Meier curves of mortality and reinfarction in the pooled population according to bolus abciximab route. AIDA STEMI: Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction; CICERO: Comparison of Intracoronary Versus Intravenous Abciximab in ST-segment Elevation Myocardial Infarction trial; M-H: Mantel-Hænszel model; IC: intracoronary; IV: intravenous; MBG: myocardial blush grade; TIMI: Thrombolysis In Myocardial Infarction
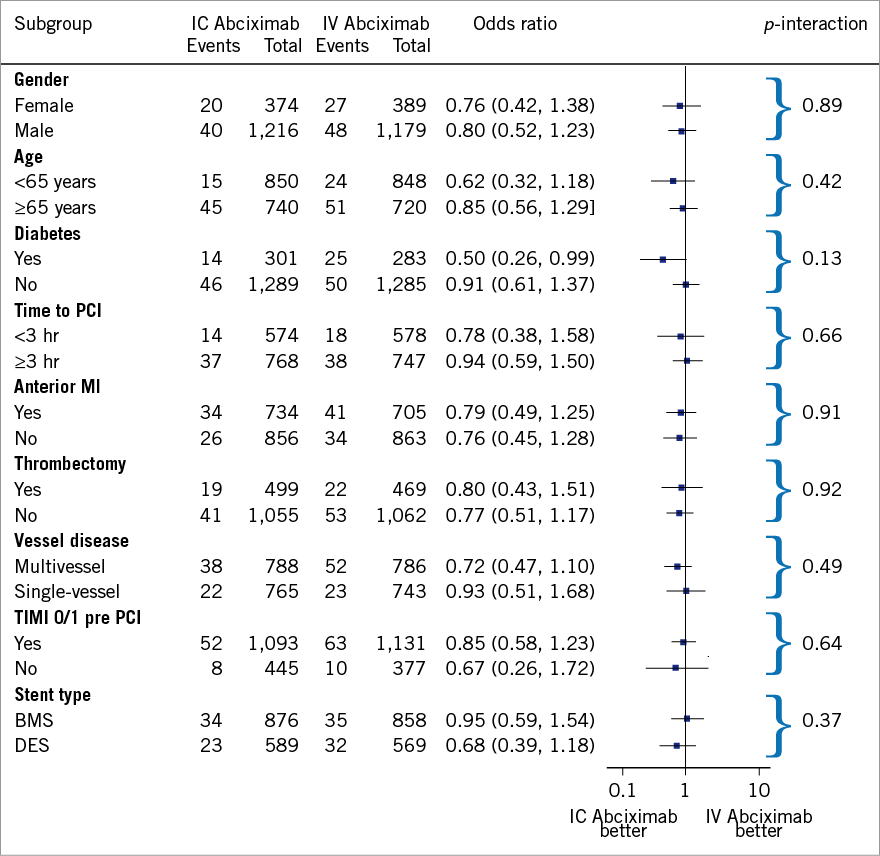
Figure 3. Results of subgroup analysis for mortality and reinfarction according to bolus abciximab route. BMS: bare metal stent; DES: drug-eluting stent; IC: intracoronary; IV: intravenous; MI: myocardial infarction; PCI: percutaneous coronary intervention; TIMI: Thrombolysis In Myocardial Infarction
As shown in Figure 4 and Figure 5, intracoronary abciximab was not associated with significant benefits in terms of death (2.5% vs. 3.2%, intracoronary vs. intravenous abciximab, respectively, OR 0.77, 95% CI: 0.51 to 1.17, p=0.22) and reinfarction (1.6% vs. 2%, intracoronary vs. intravenous abciximab, respectively, OR 0.79, 95% CI: 0.46 to 1.33, p=0.38).
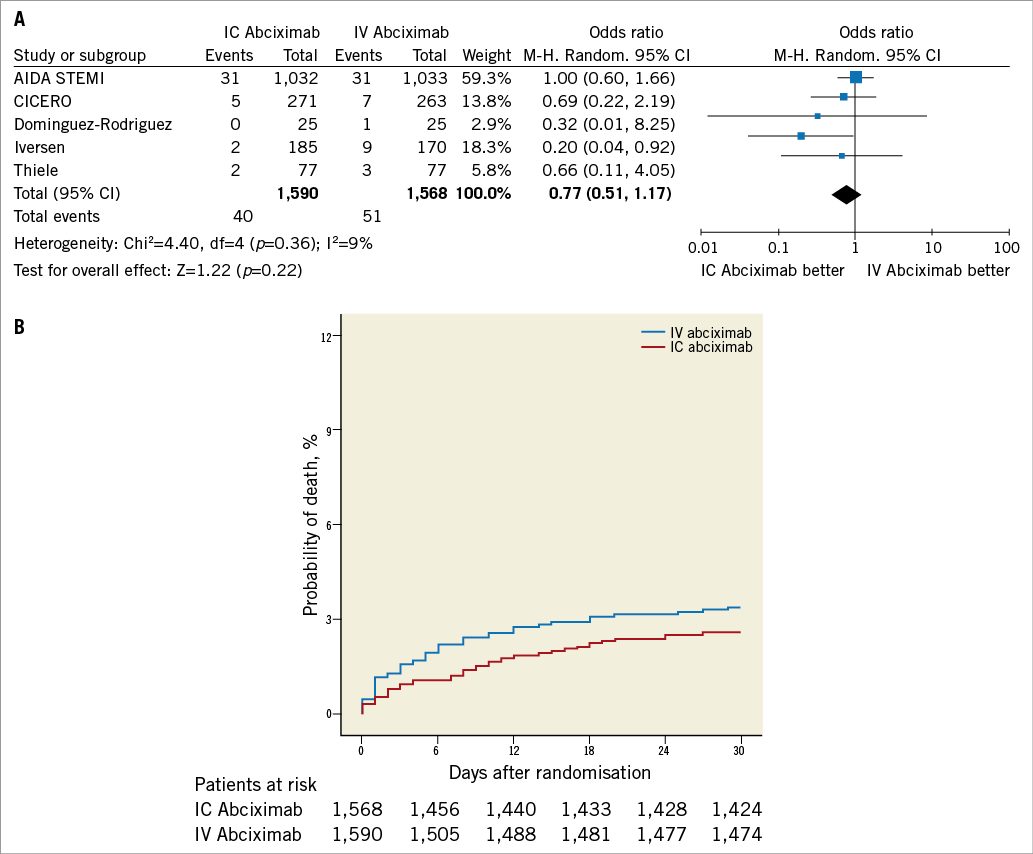
Figure 4. Analysis of mortality according to bolus abciximab route. A) Odds ratio (OR) of mortality associated with intracoronary versus intravenous bolus abciximab administration for individual trials and pooled population. B) Kaplan-Meier curves of mortality in the pooled population according to bolus abciximab route. AIDA STEMI: Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction; CICERO: Comparison of Intracoronary Versus Intravenous Abciximab in ST-segment Elevation Myocardial Infarction trial; M-H: Mantel-Hænszel model; IC: intracoronary; IV: intravenous; MBG: myocardial blush grade; TIMI: Thrombolysis In Myocardial Infarction
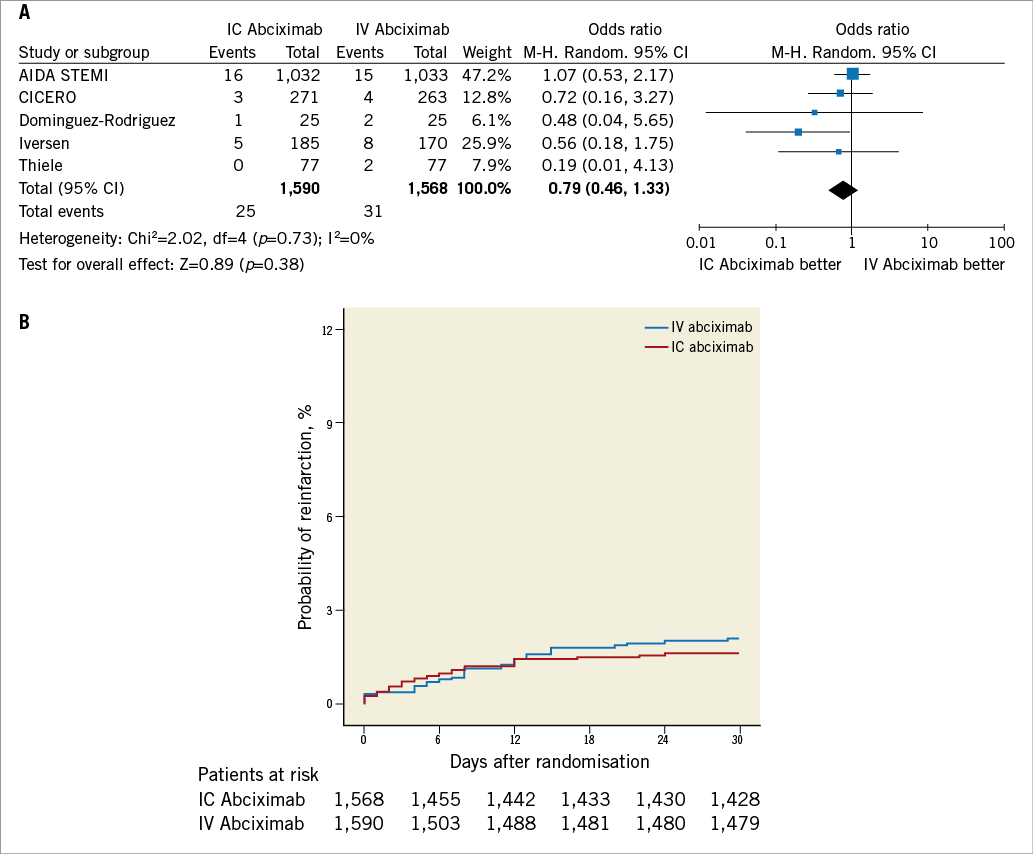
Figure 5. Analysis of reinfarction according to bolus abciximab route. A) Odds ratio (OR) of reinfarction associated with intracoronary versus intravenous bolus abciximab administration for individual trials and pooled population. B) Kaplan-Meier curves of reinfarction in the pooled population according to bolus abciximab route. AIDA STEMI: Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction; CICERO: Comparison of Intracoronary Versus Intravenous Abciximab in ST-segment Elevation Myocardial Infarction trial; M-H: Mantel-Hænszel model; IC: intracoronary; IV: intravenous; MBG: myocardial blush grade; TIMI: Thrombolysis In Myocardial Infarction
As reported in Figure 6, early definite or probable stent thrombosis was not significantly different between patients allocated to the intracoronary or intravenous arms (1.3% vs. 1.7%, respectively, OR 0.77, 95% CI: 0.43 to 1.35, p=0.36).
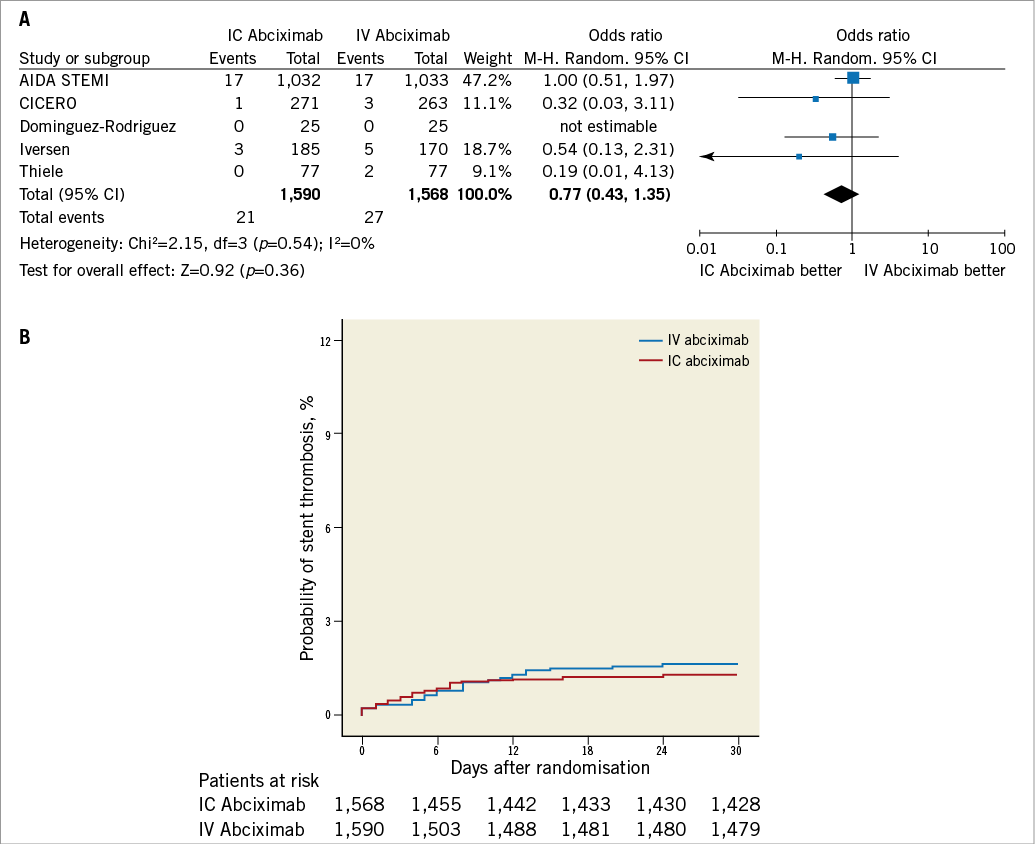
Figure 6. Analysis of stent thrombosis according to bolus abciximab route. A) Odds ratio (OR) of stent thrombosis associated with intracoronary versus intravenous bolus abciximab administration for individual trials and pooled population. B) Kaplan-Meier curves of stent thrombosis in the pooled population according to bolus abciximab route. AIDA STEMI: Abciximab Intracoronary versus intravenously Drug Application in ST-elevation myocardial infarction; CICERO: Comparison of Intracoronary Versus Intravenous Abciximab in ST-segment Elevation Myocardial Infarction trial; M-H: Mantel-Hænszel model; IC: intracoronary; IV: intravenous; MBG: myocardial blush grade; TIMI: Thrombolysis In Myocardial Infarction
REGRESSION ANALYSIS
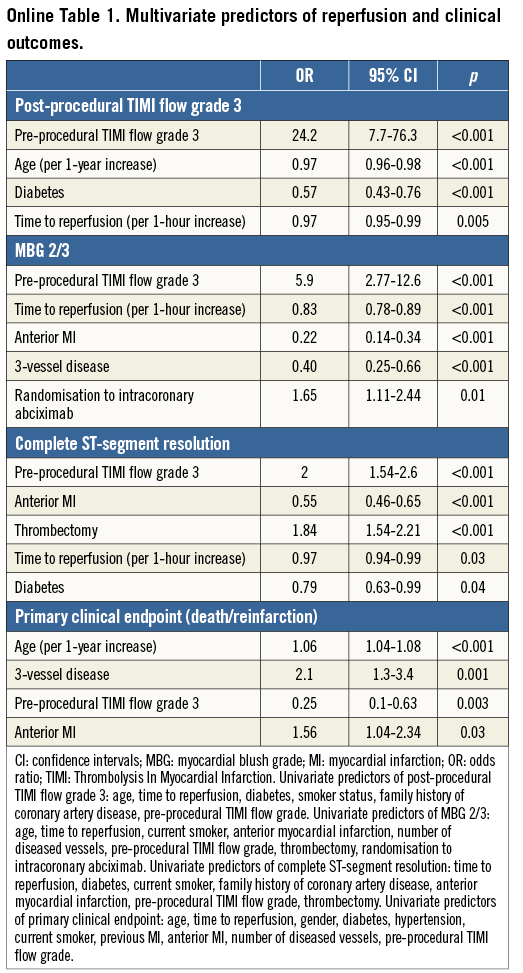
Multivariable predictors of reperfusional indices and primary clinical endpoints are listed in the Online Table 1.
SENSITIVITY ANALYSES
Sensitivity analysis performed using a Bayesian random effects model showed similar results although post-procedural MBG 2/3 was not significant (post-procedural TIMI 3 flow: OR 1.21, 95% credible interval (CrI): 0.74 to 2.38; post-procedural MBG 2/3: OR 2.03, 95% CrI: 0.05 to 77.47; complete ST-segment resolution: OR 1.25, 95% CrI: 0.57 to 3.43; mortality and reinfarction: OR 0.51, 95% CrI: 0.12 to 1.84; mortality: OR 0.67, 95% CrI: 0.17 to 1.87; reinfarction: OR 0.62, 95% CrI: 0.13 to 2.73; stent thrombosis: OR 0.69, 95% CrI: 0.14 to 2.23).
Influence analysis demonstrated that the AIDA STEMI trial negatively influenced post-procedural TIMI 3 flow, the composite of death and reinfarction, and death, since its omission resulted in a movement of the point estimate outside the 95% CI (Online Figure 1 and Online Figure 2).
Discussion
In this study we performed a pooled analysis of five randomised trials comparing intracoronary with intravenous bolus administration of abciximab in patients undergoing primary PCI. The main findings are as follows: a) reperfusion endpoints were not significantly different among patients receiving intracoronary or intravenous abciximab, with the exception of myocardial blush grade; and b) intracoronary abciximab compared to the standard intravenous route failed to improve clinical outcomes at 30-day follow-up.
The rationale for intracoronary administration of abciximab in STEMI patients derives from several experimental observations7. Collet et al20 found that abciximab is able to modify clot architecture, and Marciniak et al21 reported that high abciximab concentrations could exert a thrombus-dissolving effect through a partial displacement of platelet-bound fibrinogen. Moreover, the diminished leukocyte-dependent reperfusion injury shown with abciximab has also been attributed to its anti-inflammatory properties, such as the binding to the vitronectin receptor and the reduced expression of the activated MAC-1 receptor on leukocytes22. In addition to forming circulating aggregates with leukocytes, platelets regulate inflammation by secreting a variety of inflammatory modulators, including CD40 ligand23. Glycoprotein IIb/IIIa antagonists have been reported to inhibit the release of sCD40L and platelet aggregation initially in vitro and, later, in clinical studies24. Receptor occupancy studies showed that myocardial reperfusion improves when fewer platelet glycoprotein IIb/IIIa receptors are available for cross-linking with fibrinogen25, and the ICE (Intracoronary Eptifibatide) trial26 found that intracoronary bolus administration of eptifibatide was superior to standard intravenous treatment in achieving a high local platelet glycoprotein IIb/IIIa receptor occupancy. However, Desch et al27 recently demonstrated that, although intracoronary abciximab results in more occupied glycoprotein IIb/IIIa receptors compared with the intravenous route, these effects are no longer present 30 minutes after bolus administration. Thus, the lack of a persistently higher glycoprotein IIb/IIIa receptor occupancy over time with intracoronary abciximab may account for the negative reperfusion and clinical results of this study.
In contrast to this pooled analysis, including the AIDA STEMI trial12, the largest randomised study that enrolled 2,065 patients, previous meta-analyses9,11,28,29 suggested a mortality reduction with intracoronary abciximab administration, but only included small or medium-sized single-centre trials powered for surrogate endpoints. In this regard, larger intervention effects have been observed for single-centre than for multicentre trials30, probably because of small-study effects and higher risk of bias domains, such as sequence generation and allocation concealment. The larger intervention effect could also be due to different mechanisms for selection of a more homogenous population in single-centre than in multicentre trials, standardised interventions, and greater expertise of teams in single-centre trials31. Accordingly, in the AIDA STEMI trial12, the intravenous abciximab group had a lower (7.6%) than expected (12%) incidence of the primary endpoint, raising the issue of the enrolment of low-risk patients and the lower power of the study. In fact, using trial sequential analysis, a statistical tool for sample size calculation in a meta-analysis32, only 3,158 (16%) of the heterogeneity adjusted required information size of 19,425 patients needed to detect a 20% relative risk reduction for death, with an alpha set to 5% and power to 80%, were accrued in the current study.
Despite the fact that there was no significant difference between intracoronary and intravenous treatments with respect to the primary endpoint in the overall population, subgroup analysis revealed a significant reduction in the occurrence of death and reinfarction in diabetic patients (Figure 3). In this high-risk subgroup, intracoronary abciximab was also associated with a trend towards a lower risk of death (OR 0.59, 95% CI: 0.28 to 1.23) and early stent thrombosis (OR 0.32, 95% CI: 0.10 to 1.05). In this regard, De Luca et al10 reported a meta-regression analysis showing a significant relationship between a patient’s risk profile and mortality benefits from intracoronary abciximab administration.
Several reasons may account for the unexpected discrepancy between myocardial reperfusion as assessed by complete ST-segment resolution and MBG 2/313. MBG reflects the mechanical patency of the microvasculature and is assessed directly after PCI, while ST-segment resolution may reflect the functional status of myocardial cells and is assessed at 60 to 90 minutes after PCI. Thus, the beneficial effects of intracoronary reperfusion immediately after PCI, as mirrored by MBG, may not be present later when ST-segment resolution is evaluated. Consistent with this, the AIDA STEMI cardiac magnetic resonance substudy33 found no difference in infarct size, myocardial salvage, left ventricular function and extent of reperfusion injury in patients randomised to intracoronary abciximab. These findings are consistent with recent receptor occupancy data27.
Limitations
This study presents several limitations. First, this pooled analysis provided clinical follow-up at 30 days. Thus, long-term follow-up data are required, although it is unlikely that an improvement in late clinical outcomes of patients receiving intracoronary bolus abciximab will be observed. Second, among included studies, only the AIDA STEMI trial12 was adequately powered to evaluate the effects of intracoronary abciximab on clinical endpoints, contributing to about 65% of our study population. Third, all included trials administered the intracoronary bolus abciximab through the guiding catheter. In this regard, the INFUSE-AMI trial34 found that infarct size at 30 days was significantly reduced by intracoronary bolus abciximab delivered to the infarct lesion site via a rapid exchange therapeutic infusion balloon catheter. However, this trial compared intracoronary abciximab to placebo. Fourth, we did not systematically collect in our database bleeding events occurring throughout 30-day follow-up. Nonetheless, no significant difference in terms of major or minor bleedings was reported among included studies.
Conclusion
In conclusion, this pooled analysis did not find a superiority of intracoronary compared to intravenous bolus abciximab administration in patients with STEMI undergoing primary PCI.
| Impact on daily practice The optimal administration route of abciximab in patients with STEMI undergoing primary PCI is still controversial. In this pooled analysis of 3,158 STEMI patients enrolled in five randomised trials, intracoronary abciximab administered through the guiding catheter was not superior to the standard intravenous route at 30 days. Therefore, standard intravenous abciximab bolus should remain the preferred administration route in these patients. |
Conflict of interest statement
H. Thiele received an unrestricted research grant and minor speaker honoraria from Lilly, Germany. The other authors have no conflicts of interest to declare.
Online Appendix. WinBUGS code for Bayesian random effects meta-analyses.
Minimally informative prior distributions were used. Thus, the findings and interpretation are close to those obtained with frequentist methods. The first 10,000 iterations were discarded and results were reported as the posterior median OR with 95% credible intervals (CrI) on the basis of a further 100,000 iterations.
Model
{
for (i in 1:Nstud)
{
P[i] <- 1/V[i]
Y[i] ~ dnorm(delta[i], P[i])
delta[i] ~ dnorm(d, prec)
}
d ~ dnorm(0, 1.0E-5)
OR <- exp(d)
tau~dunif(0,10)
tau.sq<-tau*tau
prec<-1/(tau.sq)
}
Data (expressed as natural logarithm of OR and variance of OR)
Initial Values
list(d=0, tau =1, delta=c(0,0,0,0,0,0,0))
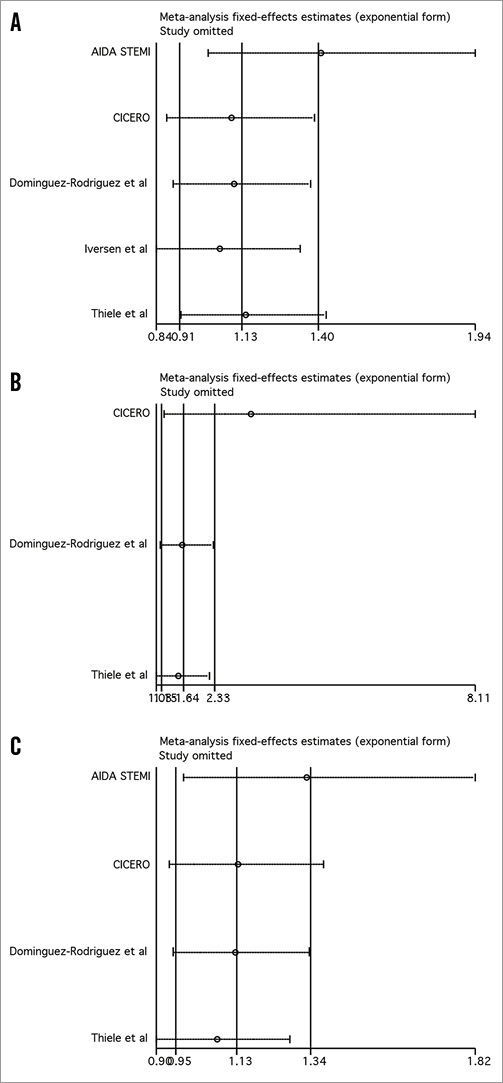
Online Figure 1. Influence analysis for post-procedural TIMI 3 flow (A), myocardial blush grade 2/3 (B), and complete ST-segment resolution (C).
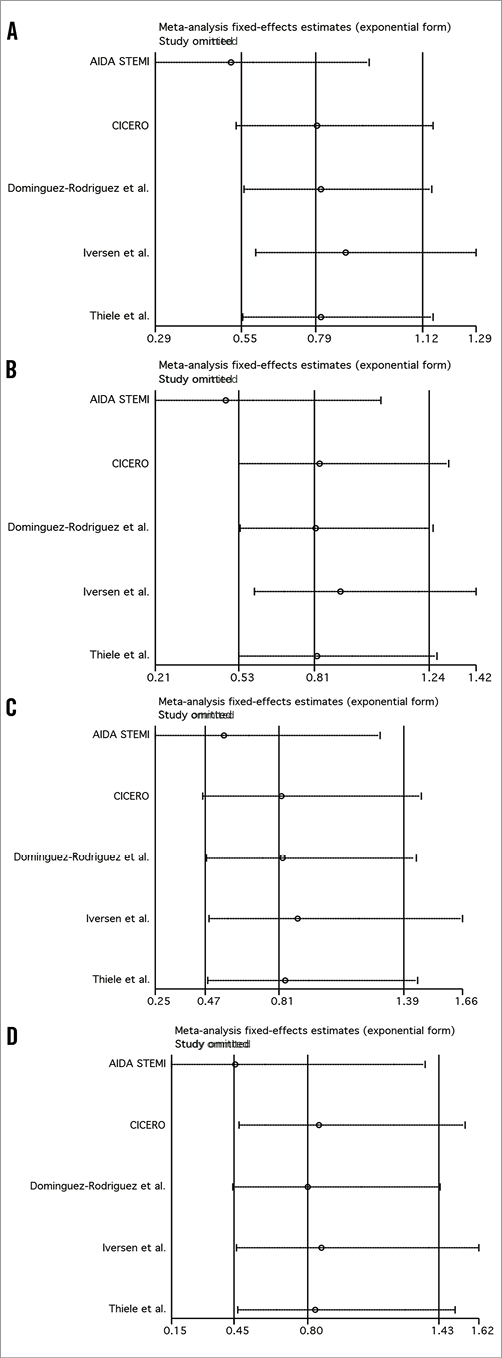
Online Figure 2. Influence analysis for the composite of death/reinfarction (A), death (B), reinfarction (C), and stent thrombosis (D).