Abstract
Aims: In primary percutaneous coronary intervention (PPCI), stenting has been shown to reduce the need for repeat target lesion revascularisation (TLR) compared to balloon angioplasty alone, but did not result in a reduction of recurrent myocardial infarction (MI) or cardiac death. Meanwhile, stent-related adverse events such as stent thrombosis continue to be of concern. Our aim was to evaluate the safety and feasibility of drug-coated balloon (DCB) angioplasty without stenting in PPCI.
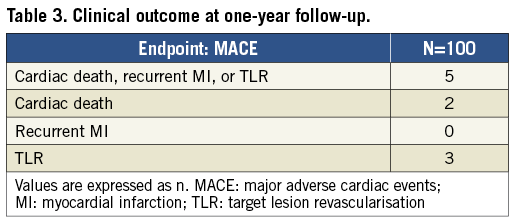
Methods and results: One hundred patients presenting with ST-elevation MI were prospectively enrolled in this pilot study. They underwent PPCI with DCB angioplasty; additional stenting was allowed only in case of type C to F coronary dissection or residual stenosis >50%. All patients were treated with i.v. bivalirudin. The primary endpoint was the composite of cardiac death, recurrent MI and TLR. A total of 59 patients received treatment with DCB angioplasty alone, whereas additional stenting was required in 41 patients. One-year clinical follow-up was completed in 98 patients. A total of five major adverse cardiac events were reported (5%). Cardiac death was seen in two patients, while three patients underwent TLR.
Conclusions: This first study of a DCB angioplasty-only strategy in the setting of PPCI showed good one-year clinical results.
Abbreviations
DAPT: dual antiplatelet therapy
DCB: drug-coated balloon
MACE: major adverse cardiac event(s)
MI: myocardial infarction
MLD: minimal lumen diameter
STEMI: ST-elevation myocardial infarction
ST: stent thrombosis
TLR: target lesion revascularisation
TVR: target vessel revascularisation
Introduction
Percutaneous coronary intervention (PCI) as the principal reperfusion strategy for ST-elevation myocardial infarction (STEMI) has been shown to improve clinical outcome compared to fibrinolytic therapy1-4. The clinical efficacy of balloon angioplasty for STEMI, however, was limited due to the relatively high degree of restenosis caused by elastic recoil and late negative remodelling5. The use of coronary stents significantly reduced the need for repeat revascularisation. Nevertheless, stenting did not result in lower rates of recurrent myocardial infarction (MI) or death, when compared to balloon angioplasty alone6-8. Subsequently, several randomised trials demonstrated that a further reduction in target lesion revascularisation (TLR) could be achieved when using drug-eluting stents (DES) as opposed to conventional bare metal stents (BMS). In agreement with studies comparing balloon angioplasty with stenting, though, none of these studies demonstrated a reduction in recurrent MI or death9-11. Meanwhile, concerns have been raised regarding an ongoing risk of stent thrombosis (ST) and/or in-stent restenosis even years after implantation, particularly in patient subsets such as STEMI12-17. Angioplasty with a drug-coated balloon (DCB) may provide a more homogeneous delivery of the antiproliferative drug, thereby limiting focal restenosis as seen in DES18. In addition, the absence of polymers and metallic scaffolding may decrease inflammation and the risk of thrombosis, and preserve coronary vasomotor response and vascular geometry14,16,19. DCB angioplasty has been shown to be an effective treatment for in-stent restenosis and small vessel disease20,21. This pilot study was designed to evaluate prospectively the feasibility and safety of a DCB angioplasty-only strategy in primary PCI (PPCI) for STEMI.
Methods
STUDY DESIGN
The PAPPA (PAclitaxel-eluting balloon in Primary Percutaneous coronary intervention in Amsterdam) pilot was designed as a prospective, observational, single-centre, non-blinded study evaluating the use of a DCB angioplasty-only strategy in STEMI. Between November 2010 and April 2011, all patients presenting with STEMI at the Onze Lieve Vrouwe Gasthuis (OLVG), Amsterdam, The Netherlands, were screened for enrolment in the study protocol. The inclusion criteria were: 1) chest pain lasting for more than 20 minutes; 2) at least 1 mm ST-segment elevation in two or more leads on the electrocardiogram, or presumably new left bundle branch block, or true posterior MI as assessed by echocardiography; 3) hospital admission within 12 hours after onset of symptoms. The exclusion criteria were: 1) active internal bleeding or a recent (< two months) history of bleeding; 2) history of intracranial disease (haemorrhage, neoplasm, arteriovenous malformation, stroke, aneurysm); 3) cardiogenic shock prior to inclusion; 4) cardiac arrest requiring intubation prior to PPCI; 5) known allergy or contraindication for aspirin, clopidogrel, prasugrel, fondaparinux and/or bivalirudin; 6) planned major surgery within six weeks; 7) stent implantation < one month prior to presentation; 8) life expectancy less than 12 months; 9) participation in another clinical trial; and 10) administration of more than 5,000 IE of unfractionated heparin before the procedure. The trial protocol required visualisation of the culprit lesion before inclusion. The target lesion had to be a de novo lesion in a native coronary artery, eligible for PPCI including stent implantation, with a reference vessel diameter ≥2.5 mm and ≤4 mm, and without severe calcification. The trial has been registered at ClinicalTrials.gov (Identifier: NCT01274728) and was approved by the institutional review board of the OLVG. All patients gave written informed consent.
MEDICATION
All patients received a loading dose of aspirin (300-500 mg) and clopidogrel (600 mg) or prasugrel (60 mg), and 5,000 IE of unfractionated heparin before PCI22,23. Additionally, all patients were treated with periprocedural intravenous bivalirudin (bolus of 0.75 mg/kg; infusion of 1.75 mg/kg during procedure) followed by a four-hour infusion at 0.25 mg/kg/hour24-27. Simultaneous use of unfractionated heparin and bivalirudin within 25 minutes was not recommended. After the procedure, antiplatelet therapy consisted of aspirin (80-100 mg o.d. for life) and clopidogrel or prasugrel (75 mg/10 mg o.d. for 12 months).
ANGIOPLASTY PROCEDURE
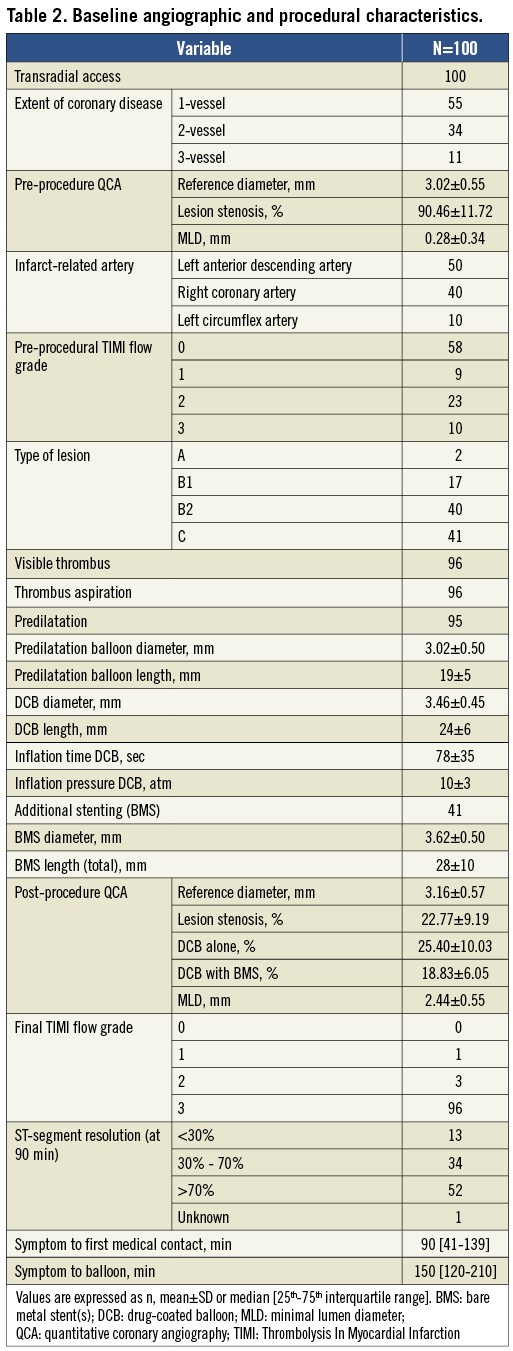
PCI was performed using standard techniques. In our institution the primary access site for PCI is the radial artery. After the administration of 100-200 µg nitroglycerine (NTG) and visualisation of the infarct-related lesion, balloon length and diameter were assessed. Thrombus aspiration as an adjunct to PPCI was recommended to reduce thrombus load and to increase coronary flow28. Predilatation with a semi-compliant balloon was mandatory to achieve an optimal lumen diameter (recommended balloon/vessel ratio ≤1). Subsequently, a paclitaxel-eluting balloon (Pantera Lux™; Biotronik, Berlin, Germany; paclitaxel dose of 3.0 µg/mm2), with a balloon-to-artery ratio >1:1, was inflated at nominal pressure for at least 60 seconds, to promote sufficient penetration of paclitaxel into the vessel wall. Additional stenting was at the discretion of the treating physician, but was only recommended in case of: 1) diameter stenosis of the treated lesion >50% by visual assessment, after DCB angioplasty; and 2) coronary artery dissection after DCB angioplasty (type C-F), leading to (threatening) vessel closure according to the National Heart, Lung and Blood Institute classification system for intimal tears29. In case of the need for additional stenting, a BMS was implanted. Stenting was performed within DCB length limits to prevent geographical mismatch between balloon dilatation and stent implantation. The number and type of BMS were at the discretion of the treating physician as well as the use of a post-dilatation balloon.
ANGIOGRAPHIC ANALYSIS
A successful DCB angioplasty was defined as: Thrombolysis In Myocardial Infarction (TIMI) coronary flow ≥2 and an estimated diameter stenosis of the treated lesion of ≤30%. DCB angioplasty with additional stenting was considered successful when TIMI flow grade was ≥2 and the estimated diameter stenosis of the treated lesion was ≤20%. Offline quantitative coronary angiography (QAngio XA 7.1; Medis, Leiden, The Netherlands) was performed by two independent investigators. In case of disagreement between the two investigators (defined as a difference of more than 15% stenosis rate), a third investigator was consulted to achieve conformity30. The target lesion was defined as the treated segment plus 5 mm immediately proximal and distal to this segment.
DATA COLLECTION AND FOLLOW-UP
At one, six and 12 months after enrolment, patients were contacted by telephone. The interview consisted of assessment of anginal status and medication regimen. In addition, major adverse cardiac events (MACE) including TLR, any target vessel revascularisation, recurrent MI, stroke, cardiac death, and any death were collected and checked in hospital records and autopsy data (when available).
STUDY ENDPOINTS AND DEFINITIONS
The primary endpoint was the composite of: 1) cardiac death; 2) recurrent MI in the target vessel area; and 3) TLR. Secondary endpoints were: 1) the need for additional stenting; 2) stent thrombosis (ST)31; and 3) major bleeding according to the non-CABG major bleeding score and TIMI major bleeding score. All deaths were considered cardiac unless a non-cardiac cause could be identified. Recurrent MI was defined as recurrence of anginal symptoms, electrocardiographic changes suggestive of MI, and an increase of troponin T or creatine kinase-myocardial band values above the upper limit of normal. R.J.S. and M.T.D. adjudicated all endpoints.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±SD in case of normal distribution or as median values (with interquartile range) if not normally distributed. Categorical variables are presented as proportions and percentages. Furthermore, we determined which angiographic variables were associated with the need for additional stenting because of coronary dissection after DCB angioplasty. In coronary balloon angioplasty, certain lesion characteristics have been shown to be associated with an increased risk of coronary dissection32. Therefore, we included the variables: calcification, presence of a significant residual stenosis after thrombus aspiration (>70% by visual assessment), lesion length, proximal vs. distal stenosis, and multivessel coronary artery disease in a binary logistic regression analysis in both univariable and multivariable models. The results were expressed as odds ratio (OR) with 95% confidence interval (CI). A p-value ≤0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
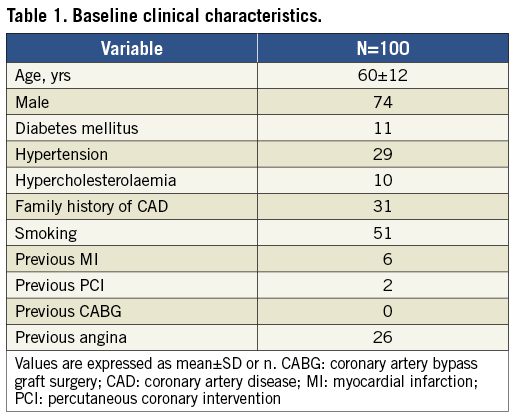
During the inclusion period, a total of 196 patients presenting with STEMI, eligible for PPCI, were screened (Figure 1). A total of 100 patients were included in the study. Baseline clinical characteristics are reported in Table 1. The mean age was 60 years, 74% were male and 11% were diabetic.
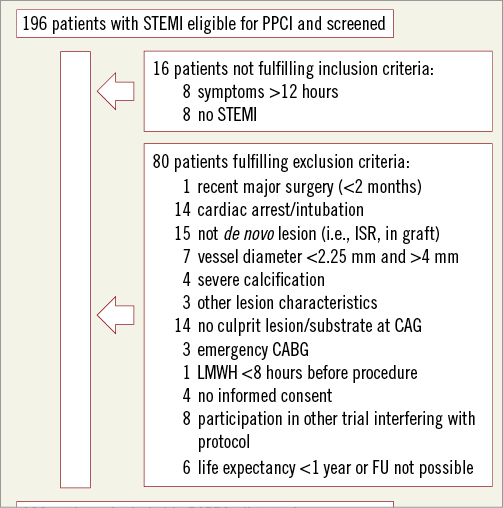
Figure 1. Flow chart of patient selection.
PROCEDURAL RESULTS
The procedural characteristics are shown in Table 2. All patients underwent PCI by transradial approach and 50% of patients underwent PCI of the left anterior descending artery. Thrombus aspiration was performed in all patients with visible thrombus (96%). Additional stenting was performed in 41 patients. Post-procedural TIMI flow ≥2 was achieved in 99 patients.
CLINICAL OUTCOME
One-year follow-up data were available for 98 patients. The results are presented in Table 3. Up to one-year follow-up, cardiac death, recurrent MI, or TLR occurred in five patients (5.0%). Two patients died due to cardiac death. Two days after discharge (five days after PPCI with DCB angioplasty alone) one patient died after cardiac arrest. The autopsy showed good patency of the target lesion and vessel. Another patient, initially treated with DCB angioplasty alone, was found dead at home at ten months. An autopsy was not performed; therefore, acute vessel closure or cardiac death could not be excluded. There were three cases of TLR. One TLR was performed due to an acute thrombosis, one hour after initial DCB angioplasty alone. A second TLR was performed in a patient with late cardiogenic shock due to high doses of antihypertensive medication. A third patient, treated with DCB angioplasty with additional stenting, underwent mitral valve repair and had additional CABG of the target vessel.
In 40 patients, additional stenting was necessary for predefined reasons, whereas one case of additional stenting because of type B coronary artery dissection was registered as a protocol deviation (Table 4). One case of acute ST was reported in a patient after DES implantation in a non-target vessel during a staged procedure.
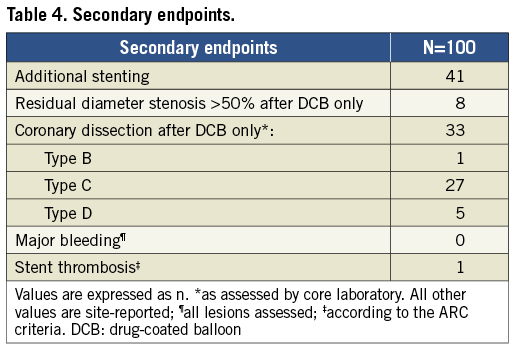
The presence of a significant residual stenosis after thrombus aspiration was the only independent predictor of the need for additional stenting after DCB angioplasty (OR 2.69, 95% CI: 1.13-6.39, p=0.026).
Discussion
This is the first study to evaluate the safety and feasibility of a strategy of paclitaxel-eluting balloon angioplasty without stenting in PPCI for STEMI. Although in this pilot study the rate of bail-out stenting was relatively high, the use of a DCB angioplasty-only strategy in the setting of PPCI seems to be a safe and feasible treatment modality. At one-year follow-up, the combined endpoint of cardiac death, recurrent MI, and TLR had occurred in only 5.0% of the study population, which is compatible with the results of DES in STEMI studies9-11. Recently, drug-coated balloon angioplasty in STEMI was examined in the DEB-AMI trial, which showed comparable clinical results33. A reliable comparison with the current pilot study, however, cannot be made since the treatment strategy differed in several respects. Most importantly, in the DEB-AMI trial stenting was required while in our study stenting was avoided. In addition, the predilatation strategy and DCB inflation time differed and may have influenced drug transfer to the vessel wall.
Mechanical reperfusion by balloon angioplasty proved to be superior to fibrinolytic therapy in terms of coronary artery patency, infarct size, and clinical outcome1-4. However, vessel patency, both early and late, was hampered by reocclusion and high restenosis rates, partly due to elastic recoil and late negative remodelling5. Stenting in PPCI, when compared with balloon angioplasty alone, resulted in a reduction of the need for target vessel revascularisation, but the use of stents did not reduce the incidence of death or recurrent MI6-8. Subsequently, the use of DES in STEMI reduced repeat revascularisation rates even more, although again without affecting death or recurrent MI9-11,34,35. On the other hand, both BMS and DES carry the risk of ST, which is a major determinant of survival and may occur even several years after implantation15,36. The risk of late ST remains a major threat after PCI and does occur despite improvements in stent design and advanced antiplatelet and antithrombotic therapy. In PPCI for STEMI, factors which may contribute to a higher risk of stent thrombosis, such as incomplete stent apposition and delayed tissue coverage, are more frequently observed compared to PCI for (un)stable angina12-17.
Balloon angioplasty has already proved to be a potent method of coronary reperfusion in the acute phase of STEMI. The deployment of a balloon with an antiproliferative coating may prevent restenosis caused by neointimal hyperplasia, and therefore a strategy of DCB angioplasty may have benefits over “plain old” balloon angioplasty. Moreover, in the current era of PPCI, improved antithrombotic medication such as prasugrel and bivalirudin may prevent early reocclusion. Finally, potential benefits of DCB angioplasty alone are in maintaining the physiologic properties of the vessel, e.g., coronary vasomotor response and vascular geometry.
During the initial phase of our pilot study, while gaining experience with this new technique, we anticipated a higher need of additional stenting. Nevertheless, the rate of bail-out stenting during the trial remained consistent, irrespective of age and infarct-related artery. Although the rate of additional stenting was relatively high, the majority of patients who were treated without a stent had good procedural and long-term clinical results.
Only five (5.0%) MACE occurred during one-year follow-up. One patient died five days after PPCI and had good patency of the target lesion and vessel at autopsy. Therefore, the probable cause of death was arrhythmic. A patient who underwent repeat PCI for an acute thrombosis after initial DCB angioplasty alone was not administered a P2Y12 inhibitor before the procedure. Another patient received repeat PCI because of cardiogenic shock caused by high doses of antihypertensive medication. During repeat intervention the target lesion was patent, with visible estimated stenosis of 40% and type B dissection. Since the patient presented with cardiogenic shock, coronary flow was inadequate, and subsequent target lesion revascularisation was performed. A third patient with valve disease underwent elective surgery and had a TLR bypass surgery. The target lesion was patent and non-significant at coronary angiography prior to surgery but, due to a new stenosis in the target vessel, coronary bypass surgery was necessary. In conclusion, four of the five MACE were presumably not related to the use of DCB angioplasty alone.
The issue can be raised as to whether it is possible to identify specific lesion and patient subsets which are suitable for a DCB angioplasty-only strategy. Acute vessel closure complicating balloon angioplasty requiring stent implantation may occur as a result of thrombosis or intimal dissection. The use of modern antiplatelet and antithrombotic therapy may well have decreased the risk of thrombotic occlusion. In our univariate and multivariate regression analysis of lesion characteristics, only the presence of a significant residual stenosis after thrombus aspiration was associated with the need for additional stenting after DCB angioplasty. In our pilot study, it is possible that the excess rate of coronary dissections, and therefore bail-out stenting, was the consequence of predilation and the use of slightly oversized DCBs (balloon-to-artery ratio >1:1)32. A DCB angioplasty-only strategy could be of especial interest in young patients with soft non-calcified lesions, diabetes and/or STEMI due to rupture of a non-significant plaque. Based on current data in patients with stable angina, dual antiplatelet therapy after PCI with DCB angioplasty is recommended for three months. This may imply that a DCB angioplasty-only strategy in STEMI would reduce the need for prolonged dual antiplatelet therapy, which might be especially beneficial for those who are at increased risk of bleeding, in case of intolerance of dual antiplatelet therapy or non-compliance.
A large randomised controlled trial with long-term follow-up data is needed to investigate further the long-term safety of a DCB angioplasty-only strategy, compared to current standard practice in PPCI for STEMI, and to identify specific patient groups that might benefit the most from a DCB angioplasty-only strategy.
Study limitations
This study has some limitations. Due to the angiographic exclusion criteria there was a selection process for included patients. Therefore, our findings do not apply to the general population of STEMI patients undergoing PPCI. Furthermore, this pilot study was designed to evaluate the feasibility and safety of a new treatment strategy, with only one year of follow-up. Long-term efficacy and safety will be studied in a randomised trial.
Conclusions
This is the first study to evaluate clinical outcome of the application of a DCB angioplasty-only strategy in the setting of PPCI for STEMI. The use of a DCB in PPCI, combined with bivalirudin and current standard concomitant medication, showed good results after one-year clinical follow-up and seems to be a safe new treatment strategy. A large randomised controlled trial is warranted to evaluate the efficacy and safety of this strategy compared to the current standard of care.
| Impact on daily practice In the setting of PPCI for STEMI, a DCB angioplasty-only strategy may be a valuable alternative for coronary stenting. Specifically, patients without significant underlying coronary artery disease or a contraindication for prolonged DAPT could benefit from this strategy, which needs further confirmation in a randomised controlled trial. |
Funding
The PAPPA pilot study is funded by the Research Department of the Division of Cardiology at Onze Lieve Vrouwe Gasthuis in Amsterdam, The Netherlands, and by Biotronik and Abbott Vascular.
Conflict of interest statement
The authors have no conflicts of interest to declare.

