
Abstract
Aims: Drug-coated balloons (DCB) may avoid stent-associated long-term complications. This trial compared the clinical outcomes of patients with non-ST-elevation myocardial infarction (NSTEMI) treated with either DCB or stents.
Methods and results: A total of 210 patients with NSTEMI were enrolled in a randomised, controlled, non-inferiority multicentre trial comparing a paclitaxel iopromide-coated DCB with primary stent treatment. The main inclusion criterion was an identifiable culprit lesion without angiographic evidence of large thrombus. The primary endpoint was target lesion failure (TLF; combined clinical endpoint consisting of cardiac or unknown death, reinfarction, and target lesion revascularisation) after nine months. Secondary endpoints included total major adverse cardiovascular events (MACE) and individual clinical endpoints. Mean age was 67±12 years, 67% were male, 62% had multivessel disease, and 31% were diabetics. One hundred and four patients were randomised to DCB, 106 to stent treatment. In the stent group, 56% of patients were treated with BMS, 44% with current-generation DES. In the DCB group, 85% of patients were treated with DCB only whereas 15% underwent additional stent implantation. During a follow-up of 9.2±0.7 months, DCB treatment was non-inferior to stent treatment with a TLF rate of 3.8% versus 6.6% (intention-to-treat, p=0.53). There was no significant difference between BMS and current-generation DES. The total MACE rate was 6.7% for DCB versus 14.2% for stent treatment (p=0.11), and 5.9% versus 14.4% in the per protocol analysis (p=0.056), respectively.
Conclusions: In patients with NSTEMI, treatment of coronary de novo lesions with DCB was non-inferior to stenting with BMS or DES. These data warrant further investigation of DCB in this setting, in larger trials with DES as comparator (ClinicalTrials.gov Identifier: NCT01489449).
Introduction
Andreas Grüntzig introduced percutaneous transluminal coronary angioplasty (PTCA) in 19771. The next important step in coronary percutaneous transluminal intervention was the development of bare metal stents (BMS), reported for the first time in 19872, initially to treat flow-limiting dissections. Later it became apparent that stents resulted in better acute outcomes and reduced the restenosis rate by about 10% in absolute terms compared to PTCA only3. However, the implantation of coronary stents was initially complicated by an unacceptably high rate of acute and subacute vascular closure. With the introduction of dual platelet aggregation inhibition in the mid 1990s, stent implantation became a safe procedure4,5. The still high restenosis rate with BMS was finally able to be reduced by local drug delivery from drug-eluting stents (DES)6. While DES of the first generation had increased thrombotic occlusion rates compared to BMS, this disadvantage was overcome in DES of the second generation. However, in long-term observational studies, the short- and medium-term benefit of stents over angioplasty was reversed. Patients treated with BMS in the course of a myocardial infarction showed very late thrombotic vascular occlusions and myocardial infarctions after an average of nine years, more than twice as often as patients treated with PTCA alone7. Furthermore, newer-generation DES also show a slight but linear increase in cardiovascular events which, according to current knowledge, appears not to plateau over time8. This has been suggested to be due to accelerated neoatherosclerosis9.
Furthermore, interventional treatment of acute coronary syndromes is associated with an increased rate of acute and subacute stent thrombosis when compared with stable coronary heart disease. Therefore, the concept of avoiding permanent implants may be especially attractive for patients with acute coronary syndrome to prevent stent-associated acute and long-term complications. Drug-coated balloons (DCB) fulfil the requirements of “leaving nothing behind” to avoid stent-associated events. Small randomised studies10 and registries11,12,13 have confirmed the safety and efficacy of the “DCB only” concept in the treatment of coronary de novo disease. Recently, two trials with primary clinical endpoints have been published for small coronary vessels (the BASKET-SMALL 2 study14) and in patients with high bleeding risk (the DEBUT study15). However, no randomised controlled trial on this concept has been published in patients with acute coronary syndrome. The aim of this prospective, randomised, controlled multicentre trial was to compare the clinical outcome of patients with non-ST-elevation myocardial infarction (NSTEMI) treated either by DCB or by stent.
Methods
STUDY DESIGN
Two hundred and ten patients with NSTEMI were enrolled in a randomised, controlled, non-inferiority multicentrre trial comparing a paclitaxel iopromide-coated DCB (SeQuent® Please and SeQuent® Please NEO, coated with 3 µg paclitaxel/mm² of balloon surface; B. Braun Melsungen AG, Berlin, Germany) with primary stent treatment (ClinicalTrials.gov Identifier: NCT01489449).
PATIENTS
The main inclusion criterion was clinical presentation with an NSTEMI defined by ischaemic symptoms (angina pectoris) >30 minutes, last symptoms within 72 hours before randomisation, positive cardiac troponin T, I, or hs-troponin above the 99th percentile, and an identifiable culprit lesion without angiographic evidence of large thrombus with intended early percutaneous coronary intervention (PCI).
PROCEDURES
After assessment of inclusion and exclusion criteria, patients were randomly assigned to undergo primary stent implantation or use of a DCB after lesion preparation according to the DCB Consensus Group recommendations16. The trial was initiated in December 2012, when BMS were still recommended in the setting of non-ST-elevation acute coronary syndrome17. During the course of the study, the investigators agreed to use new-generation limus-eluting DES.
The primary endpoint was target lesion failure (TLF; combined clinical endpoint consisting of cardiac or unknown death, myocardial reinfarction, and target lesion revascularisation) after nine months. Secondary endpoints included total major adverse cardiovascular events (MACE) consisting of all-cause mortality, myocardial infarction, target lesion revascularisation, stroke, or PCI at other vessels. Furthermore, individual clinical endpoints were defined as secondary endpoints. All endpoints were defined according to the Academic Research Consortium (ARC) definitions18. For further details please refer to Supplementary Appendix 1 and the CONSORT checklist (Supplementary Appendix 2).
Results
PATIENTS
Two hundred and ten patients with NSTEMI were enrolled in this randomised study between December 2012 and January 2017. Mean age was 67±12 years, 67% were male, 62% had multivessel disease, and 31% were diabetics. One hundred and four patients were randomised to DCB treatment, 106 to stent treatment. Table 1 summarises the baseline clinical data. In total, 243 lesions were treated, 123 in the DCB group and 120 in the stent group. In the stent group, 56% of patients were treated with BMS, 44% with current-generation DES. In the DCB group, 85% of patients were treated with DCB only whereas 15% underwent additional stent implantation. Two lesions in the DCB group were treated with plain old balloon angioplasty (POBA) only, since no study device as well as no crossover stent could be advanced to the lesion. Procedural data are presented in Supplementary Table 1. No differences in length of hospital stay and medical treatment at discharge were observed between the groups (Supplementary Table 2).
PRIMARY ENDPOINT
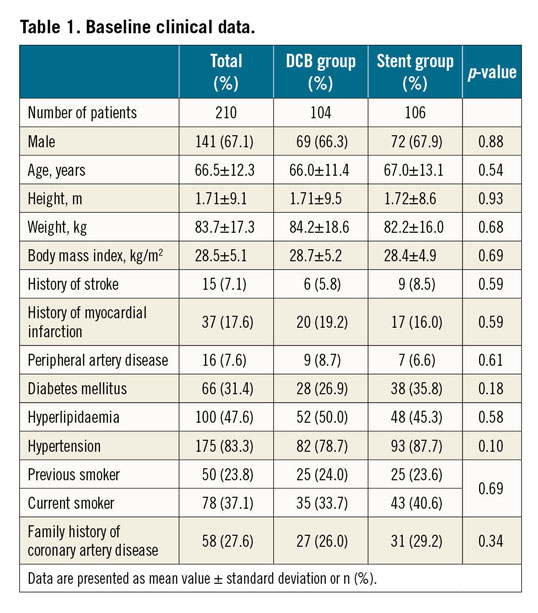
During a follow-up of 9.2±0.7 months, the TLF rate was 3.8% in patients randomised to DCB treatment versus 6.6% in those randomised to primary stenting (intention-to-treat; p=0.53; difference −0.03, 97.5% confidence interval [CI]: −0.1057 to 0.0506). Non-inferiority of DCB versus stent was able to be demonstrated according to Farrington and Manning with a non-inferiority level of −0.07 (90% CI: −0.0315 to 0.0867), a proportion difference of 0.0276, and a significance level of <0.0033 (Table 2, Figure 1). There was no significant difference in TLF rates in the per protocol analysis (Figure 2) or between BMS and current-generation DES.
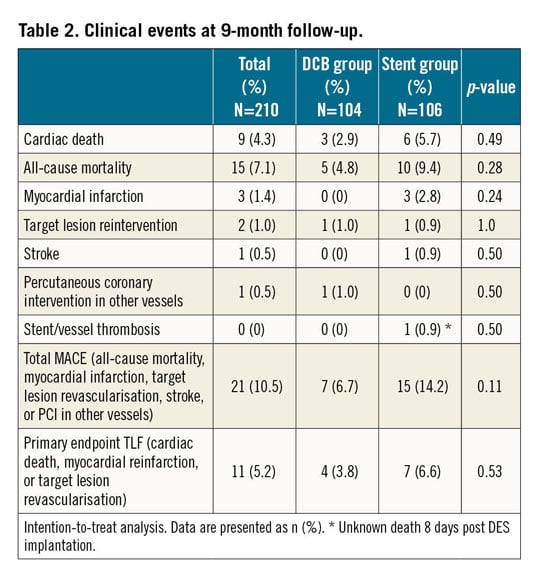
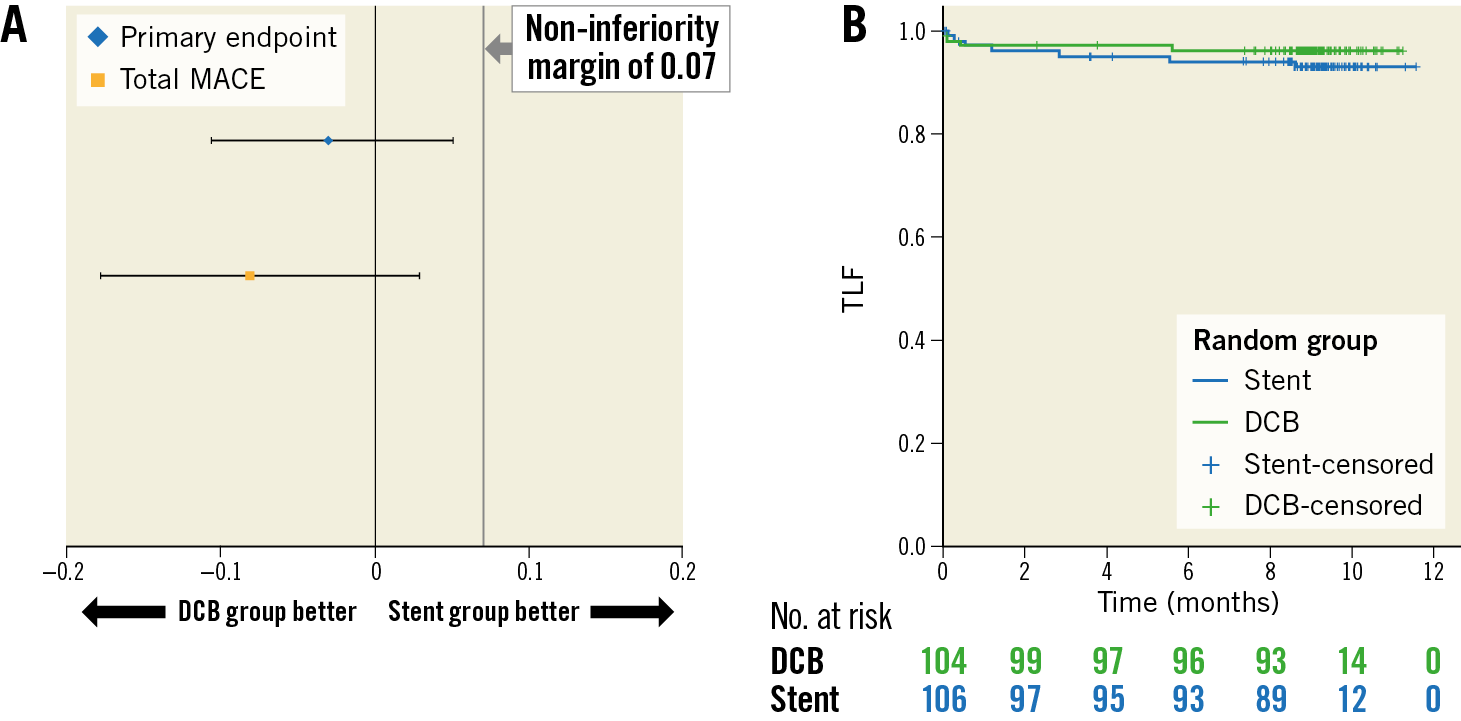
Figure 1. Primary endpoint. A) Test for non-inferiority, intention to treat. Ratio of event rates (97.5% CI) for the primary endpoint target lesion failure (TLF consisting of cardiac death, myocardial reinfarction, or target lesion revascularisation) and total major adverse cardiac events (total MACE; all-cause mortality, myocardial infarction, target lesion revascularisation, stroke, or PCI at other vessels). Confidence interval for TLF nine months: difference −0.03, 97.5% CI: −0.1057 to 0.0506. Confidence interval for total MACE nine months: difference −0.08, 97.5% CI: −0.1775 to 0.0291. B) Kaplan-Meier analysis of the primary endpoint TLF at nine months (intention to treat). P (log-rank)=0.360.
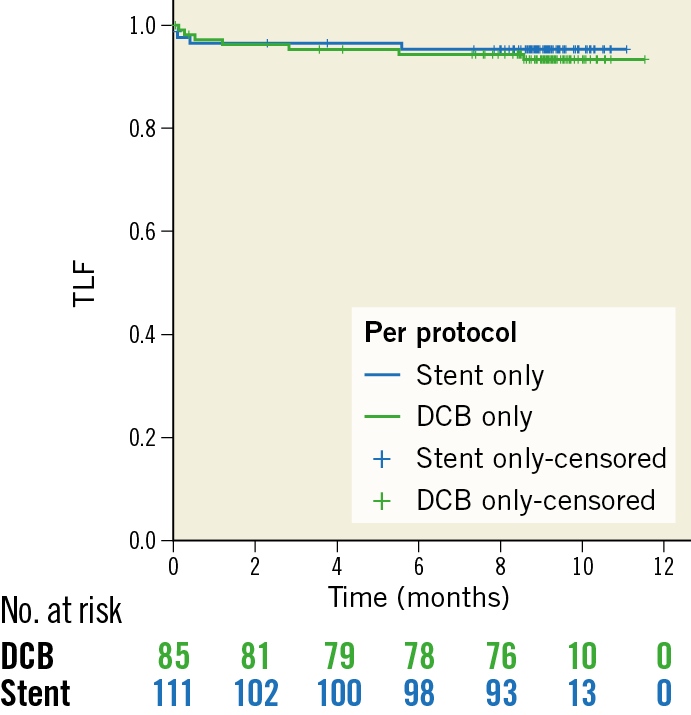
Figure 2. Kaplan-Meier analysis of the primary endpoint target lesion failure (TLF consisting of cardiac death, myocardial reinfarction, or target lesion revascularisation) at nine months (per protocol). P (log-rank)=0.615.
SECONDARY ENDPOINTS
Rates of death (4.8% vs 9.4%), myocardial infarction (0 vs 2.8%), target lesion reintervention (1.0% vs 0.9%), stroke (0 vs 0.9%), and PCI at other vessels (1.0% vs 0) did not differ significantly between patients randomised to DCB or stent treatment, respectively. In the DCB group, no acute or subacute thrombotic stent or vessel occlusions occurred. In the stent group, one patient died eight days after DES implantation when at home (unknown death).
The total MACE rate was 6.7% for DCB versus 14.2% for stent treatment (p=0.11) (Figure 3), and 5.9% versus 14.4% in the per protocol analysis (p=0.056) (Table 3, Supplementary Figure 1), respectively. No significant differences were observed between BMS and DES, whereas both treatments had higher event rates compared to “DCB only”; however, the latter difference was not statistically significant (Supplementary Table 3, Supplementary Figure 2, Supplementary Figure 3).
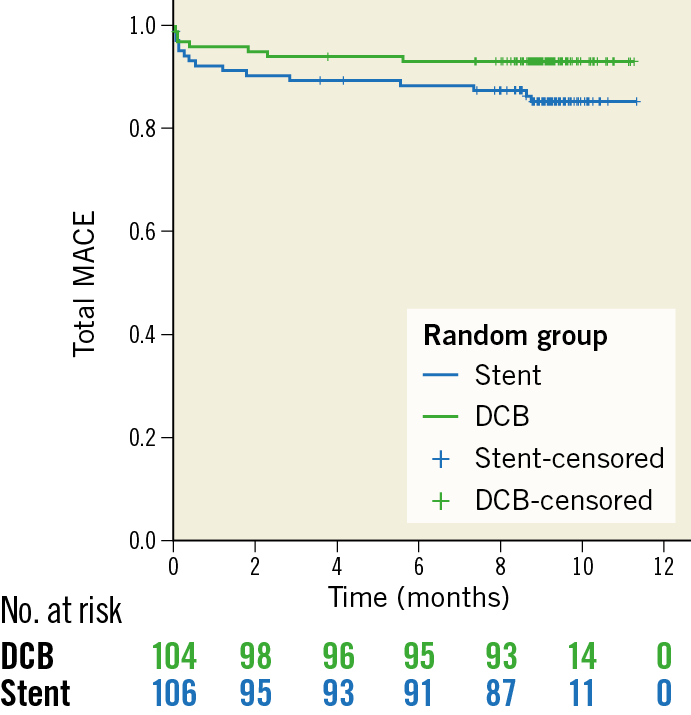
Figure 3. Kaplan-Meier analysis of total MACE (all-cause mortality, myocardial infarction, target lesion revascularisation, stroke, or PCI at other vessels) at nine months (intention to treat). P (log-rank)=0.082.
Discussion
Non-ST-elevation acute coronary syndrome is the most common trigger for invasive coronary diagnostics and interventions worldwide. Despite the lack of larger randomised trials on the preferred interventional technique, DES are regarded as the standard of care in most countries19. However, until now no single randomised study has demonstrated the superiority of DES compared with BMS or even POBA in the prevention of death and recurrent myocardial infarction7.
The increased risk of thrombotic complications in acute coronary syndrome is a striking argument to avoid permanent implants. In recent years, there has been growing interest in the development and investigation of bioresorbable scaffolds. The promise of preventing medium- and long-term complications associated with leaving a foreign metallic stent within the vessel, by avoiding permanent implants, is indeed conceptually very attractive. However, the price to be paid in the form of early and late thrombotic complications has so far been too high20.
Drug-coated balloons cannot replace stents or scaffolds in all clinical situations. However, if used in accordance with the recommendations of the DCB Consensus Group16,21, stent implantation might be avoided in many lesions. The main contraindications for DCB treatment are flow-limiting dissections and an unsatisfactory initial lumen gain. Contrary to the fears of some interventional cardiologists who were trained against the background of primary stent implantation, the “DCB only” procedure appears to be safe. In the Swedish SCAAR registry, for example, in almost 2,400 propensity-matched patients, not only was the rate of thrombotic vascular occlusion after DCB significantly lower after five years, but also and above all the acute occlusion rate due to DCB treatment was reduced compared with current-generation DES12. The present trial supports these findings since there were no cases of acute vessel closure in DCB-treated patients, which is in line with the findings from the BASKET-SMALL 2 trial in coronary arteries smaller than 3 mm14 and the DEBUT trial in patients with high bleeding risk15.
This finding seems surprising, but it is very plausible. The early reduction of vascular occlusions after stent implantation is related to the fixation of flow-limiting dissections, which are rare. However, the prevention of acute and subacute stent thrombosis is based mainly on the initiation of dual antiplatelet therapy4,5. For balloon angioplasty alone, the impact of this drug treatment has never been systematically investigated. The important first procedural step in the “DCB only” concept is to achieve sufficient lumen gain by adequate preparation of the lesion and to detect incident flow-limiting dissections. Inhibition of restenosis is a consequence of local drug application, which can also be achieved with DCB treatment. The special feature of the “DCB only” treatment is that it results in lumen enlargement after a few months post treatment22,23, which can be considered a type of vascular restoration. This phenomenon is the basis for accepting a certain residual stenosis during the intervention. Of note, stent-based therapies do not show this effect.
Interestingly, patients in the stent group who had received a BMS showed similar event rates to those who had received the new-generation DES. The somewhat lower reintervention rate of DES was not sufficient to achieve a significant advantage over BMS. The 12-month duration of dual antiplatelet therapy in all patients may have played a role here, regardless of the stent type used. These results are in accordance with a current Cochrane meta-analysis in 12,503 patients presenting with acute coronary syndrome, in which there was no difference in survival between BMS and DES, but differences in the incidence of TLR were found24.
The results of the present study support the safety of coronary intervention without stent implantation in patients with an increased thrombotic risk. After nine months, there was no statistically significant difference in all relevant clinical endpoints between primary stent therapy and DCB only. This means that, unlike bioresorbable stents, this approach does not increase the event rate within the first few months by avoiding permanent implants. However, superiority for DCB only may only be demonstrated in a longer-term follow-up. Patients in this study will be followed up for up to five years.
Limitations
Patients with NSTEMI represent a heterogeneous patient population. Using the DCB concept, lesions with a high thrombus burden were excluded because the concept of a single short-term drug application probably makes little sense here. The decision for inclusion in the study was always made immediately after diagnostic coronary angiography and before PCI. Following the presentation of the concept of DCB only in 201116, several studies in different indications were initiated to investigate this new concept in de novo lesions with a primary clinical endpoint. For small coronary vessels there was the BASKET-SMALL 2 study14, for high bleeding risk the DEBUT study15, and for ACS the PEPCAD NSTEMI study. When conducting these trials, it was difficult to find centres that wanted to accept this new and untested concept. In the participating centres it was usually the case that only one or two operators were willing to include patients at all. This explains the long recruitment time in some of the studies. In spite of this limitation, all three studies have delivered convincing results showing the safety and efficacy of DCB only in studies with primary clinical endpoints.
Based on the data available at the initiation of the study, BMS were initially used in the control group. Following the general recommendation of DES in the guidelines, the use of current-generation DES was recommended after inclusion of about half of the patients. Exclusive use of DES in the comparator arm would have been more favourable. Unfortunately, the study does not have sufficient statistical power for a subgroup comparison. Furthermore, there was no routine angiographic follow-up in the study, so event rates could be underestimated. The power of the study is limited regarding its primary endpoint and also the non-inferiority margin selected. Furthermore, event adjudication was carried out by local investigators without a centralised and independent clinical events committee.
Nevertheless, our findings are in concert with results of previous registries11,12,13,25 and trials comparing DCB with stents in small coronary vessels10,26,27 and normal-sized vessels in patients at high risk of bleeding15. In the BELLO study, for example, there was no difference between DCB and DES in the clinical events after one year in small coronary vessels10, but after three years there was a significant advantage for DCB therapy in terms of reduction of major adverse events28. The recently presented DEBUT study compared 210 patients treated with “DCB only” versus BMS in patients at high risk of bleeding. The frequency of major adverse events after nine months was 12.4% for the BMS, while only 1.9% events occurred after DCB15.
Conclusions
In conclusion, treatment of coronary de novo lesions with DCB was non-inferior to stenting with BMS or DES. These data warrant further investigation of DCB in this setting, in larger trials with DES as comparator. Longer-term follow-up will scrutinise whether avoiding permanent implants is advantageous over traditional stent therapy in certain patients.
|
Impact on daily practice DCB use for ISR therapy has a IA recommendation in the ESC guidelines. So far, there is no such recommendation for the treatment of de novo stenoses. For de novo lesions, randomised trials with primary clinical endpoints have demonstrated the safety and efficacy of DCB for small coronary vessels (BASKET-SMALL 2 trial), patients with high risk of bleeding (DEBUT trial) and now also patients with NSTEMI (PEPCAD NSTEMI trial). |
Funding
Financial support was provided by B. Braun Melsungen AG, Berlin, Germany.
Appendix. Study collaborators
Johannes Brachmann, MD; Klinikum Coburg, Coburg, Germany; Anne Bärisch, MD; Vivantes Klinikum im Friedrichshain, Berlin, Germany.
Conflict of interest statement
B. Scheller is a shareholder of InnoRa GmbH, Berlin, and was named as co-inventor on patent applications submitted by Charité University Hospital, Berlin, Germany. M.A. Ohlow has received research support from Translumina and proctoring fees and travel support from Biosensors. T.K. Rudolph has received speaker’s honoraria from Abbott Vascular. M. Böhm is supported by the Deutsche Forschungsgemeinschaft (SFB TTR 219, S-01) and has received research support from Medtronic and St. Jude. R. Degenhardt has received research support from B. Braun. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

