
Primary percutaneous coronary intervention (PCI) is the evidence-based standard of care for patients with acute ST-segment elevation myocardial infarction (STEMI)1. Despite routinely restoring coronary flow2, failed myocardial reperfusion, or microvascular obstruction, affects approximately half of patients2, increasing the risk of death and heart failure in the longer term2,3. Primary PCI should not be considered successful when microvascular obstruction has occurred.
Microvascular obstruction is caused by intrinsic and extrinsic vascular processes (Figure 1). The intrinsic problems include impaired flow, microvascular thrombosis, distal embolisation of thrombus/atheroma, vasospasm and endothelial damage. The extrinsic problems include microvascular compression due to oedema and inflammation. Some of the problems, e.g., inflammation, are both intrinsic and extrinsic to the microcirculation. Endothelial damage impairs capillary integrity causing oedema and eventually haemorrhagic transformation within the infarct core. Microvascular obstruction precedes myocardial haemorrhage and, while it may be reversible, myocardial haemorrhage is not2. Iron deposition within the infarcted myocardium drives inflammation4, increasing the likelihood of ventricular arrhythmias, adverse remodelling2 and adverse cardiac events2,3. Microvascular obstruction is a therapeutic target in practice guideline recommendations1; however, there are no evidence-based treatments for this problem. Clinical trials of cardioprotective interventions have not proven beneficial5,6, and intracoronary vasodilator therapy with high-dose (2 mg) adenosine was harmful (REFLO-STEMI)7.
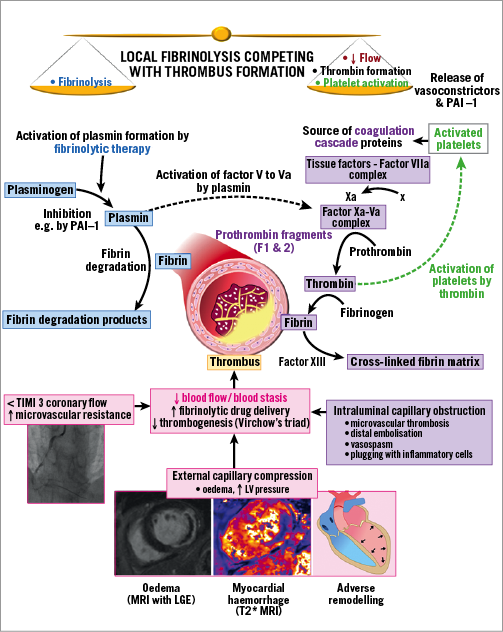
Figure 1. Competing processes involved in local fibrinolytic therapy. LGE: late gadolinium enhancement; LV: left ventricular; PAI-1: plasminogen activator inhibitor-1
Thrombus burden is associated with adverse outcomes after primary PCI, driven, in part, by intraprocedural thrombotic events8. Microvascular thrombosis within viable myocardium is causally implicated in the progression from microvascular obstruction to myocardial haemorrhage if microvascular reperfusion is not achieved9. Therefore, a pharmacoinvasive strategy involving low-dose intracoronary fibrinolytic therapy to reduce thrombus burden and reperfuse the microcirculation is theoretically warranted. Sezer et al10 gave adjunctive low-dose streptokinase (250 kU) at the end of primary PCI; this approach appeared to improve myocardial reperfusion and reduce infarct size.
In this issue of EuroIntervention, Morales-Ponce et al11 report a randomised, active controlled pilot trial of adjunctive treatment with either intracoronary low-dose tenecteplase or the glycoprotein IIb/IIIa inhibitor abciximab during primary PCI.
From 2012 to 2016, 76 patients (78% male, 87% with Thrombolysis In Myocardial Infarction [TIMI] flow grade 0 or 1) were randomised to intracoronary tenecteplase (1/5th systemic dose) infused over three minutes before stent implantation, or intracoronary abciximab (0.25 mg/kg bolus) followed by an intravenous infusion (0.125 mcg/kg/min for 12 hours) during primary PCI. Angiography was repeated at 48 hours to assess the corrected TIMI frame count and myocardial perfusion grade. The primary endpoint was infarct size assessed by cardiac magnetic resonance imaging (MRI) at four months.
The abciximab group had a lower corrected TIMI frame count (median 14.1 [IQR 9.4-17.1]) than the tenecteplase group (18.2 [10.0-28.2], p=0.02), and a higher proportion of patients with TIMI myocardial perfusion grade 2/3 (90.3% vs. 67.7%, p=0.03). Major cardiac and cerebrovascular event rates did not differ; however, 2/38 patients in the tenecteplase group experienced subacute stent thrombosis. Infarct size was lower in the tenecteplase group, but this difference was not statistically significant (17.0 g [9.6-27.5] vs. 21.1 g [11.3-35.0], p=0.33). The authors highlight potential pro-coagulant effects of intracoronary tenecteplase as given in this trial.
The study’s strengths include the research question, adoption of repeat invasive coronary angiography at 48 hours, and contrast-enhanced cardiac MRI. The four-year enrolment period points to the challenges of undertaking a study of this kind. The limitations include the open design using unmasked interventions and suboptimal inhibition of platelet activation because clopidogrel was used rather than prasugrel or ticagrelor. This point is important given the possibility of thrombin activation by tenecteplase (Figure 1). Glycoprotein IIb/IIIa inhibitor therapy was given as the intervention in half of the participants rather than as a bail-out treatment for thrombotic complications1. The four-month time point for MRI ruled out the possibility of assessing microvascular obstruction since it commonly resolves over time. Finally, a control group would have permitted assessments against a placebo. Presumably this was not feasible. The sample size is not a limitation since the study was designed as a pilot.
There are four other trials of intracoronary reduced dose lytic therapy, including T-TIME (NCT02257294), STRIVE (NCT03335839) and the smaller OPTIMAL trial (NCT02894138) using alteplase, and RESTORE-MI (ACTRN12618000778280) using tenecteplase. We recently reported the results of T-TIME, a phase 2 clinical trial of low-dose adjunctive intracoronary fibrinolysis with alteplase in reperfused STEMI12. The objective was to conduct a safety, efficacy and mechanisms evaluation within the context of a trial that was sufficiently large (n=440) to give definitive results using surrogate outcomes. Patients with an occluded infarct-related artery and/or a high thrombus burden at initial angiography were enrolled, since these characteristics are risk factors for microvascular obstruction. By targeting thrombus within the infarct-related artery and microcirculation with fibrinolytic therapy the aim was to restore microvascular blood flow at the earliest point after coronary reperfusion. In T-TIME microvascular obstruction was not reduced by alteplase.
The evidence from the study by Morales-Ponce et al11 and T-TIME calls into question the primacy of thrombosis in the pathophysiology of failed myocardial reperfusion. One learning point from these trials is that intracoronary fibrinolytic therapy (and potentially related interventions in the future) should be scheduled at the end of PCI when coronary flow is optimised. This approach was adopted by Sezer et al10 and is proposed in RESTORE-MI and STRIVE. Given the unmet therapeutic need presented by microvascular obstruction, more research is needed.
Acknowledgements
We thank Dr Campbell Tait, Consultant Haematologist, Glasgow Royal Infirmary, Glasgow, United Kingdom.
Funding
This editorial describes research that was supported by a grant from the National Institute for Health Research Efficacy and Mechanism Evaluation (NIHR EME) programme (reference: 12/170/45) and in-kind support from Boehringer Ingelheim. A. Maznyczka is funded by a Fellowship from the British Heart Foundation (FS/16/74/32573).
Conflict of interest statement
The authors have no conflicts of interest to declare.

