
The widespread application of coronary reperfusion strategies in order to limit infarct size is based on the open artery hypothesis formulated by Eugene Braunwald in the 1970s1,2. As a result, the in-hospital mortality declined and the one-year clinical outcome after a first ST-elevation myocardial infarction (STEMI) has improved dramatically in recent decades3. Although primary percutaneous coronary intervention (PPCI) creates a complete recanalisation of the occluded epicardial coronary artery in almost all STEMI patients, this does not result in an effective myocardial reperfusion in about 35% of patients4. This condition, known as the slow or no-reflow phenomenon, has been related to ischaemia and reperfusion injury affecting both the myocardial microcirculation and cardiomyocytes. Distal embolisation of atherothrombotic material from the culprit lesion, either spontaneous or provoked by the intracoronary manipulation of guidewires and devices during the mechanical recanalisation procedure, creates another mechanism for no-reflow (Figure 1). Absence or incomplete resolution (<50 to 70%) of the ST-segment elevation is an established marker of no-reflow which predicts a worse prognosis after both thrombolysis5 and mechanical recanalisation6. Approximately one third of the patients with TIMI flow grade 3 and myocardial blush grade 2 to 3 do not exhibit a sufficient ST segment resolution7.
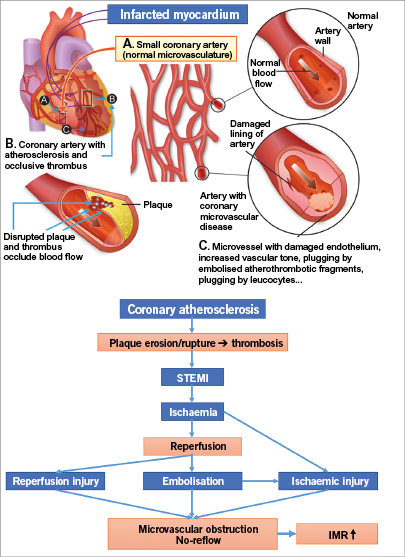
Figure 1. Pathophysiology of the “no-reflow” phenomenon. Adapted from Anderson RD & Pepine CJ13.
In the current issue, De Maria et al present a new scoring system that can be used at the time of coronary intervention to predict the likelihood of downstream microvascular impairment following mechanical reperfusion in patients with STEMI8. The newly derived ATI score is based on the age of the patient, the angiographic thrombus burden of the infarct-related artery and on the measurement before stenting of the index of microcirculatory resistance (IMR). An ATI score ≥4 was associated with a 95.1% risk of a final post-intervention IMR >40, whilst an ATI score <2 was not associated with a final IMR >40. An elevated IMR >40 measured immediately after PPCI for STEMI has been shown to be a strong long-term predictor of death and rehospitalisation for heart failure9. The new ATI score therefore appears to be a promising tool, on the one hand to designate STEMI patients on the cathlab table as low-risk with minimal microvascular injury in whom early mobilisation and discharge can be considered, and on the other hand to identify a high-risk subgroup with important post-procedural microvascular impairment in whom intensive cardiac care surveillance is necessary.
The present study further confirms that, even when potent antithrombotic therapies and modern interventional techniques are used, one third of the STEMI patients still develop a major microvascular impairment (IMR >40) in the infarct-related perfusion territory post PPCI. Although ischaemia and reperfusion injury undoubtedly contribute to the pathogenesis of this post-procedural microvascular impairment and its associated increased infarct size, we must not underestimate the importance of distal embolisation. In the present study, the thrombus score was the strongest predictor of post-procedural microvascular impairment, whereas a high pre-stenting IMR was only a weak predictor (OR 2.82 vs. 1.03). Pre-stenting IMR is most probably the composite result of pre-procedural ischaemic injury of the coronary microcirculation and of already present distal embolisation triggered by thrombus aspiration and/or balloon predilatation, which were performed in many patients in this study. Hence, the important role of procedure-related distal embolisation in the pathophysiology of the microvascular dysfunction occurring during mechanical reperfusion should be highlighted. Wiring of a heavily thrombus-laden lesion, occlusive or not, predilatation and stent delivery can all inevitably lead to distal embolisation that will contribute to microvascular plugging and dysfunction10. In a previous study, a large thrombus burden in the setting of PPCI for STEMI was associated with larger myocardial damage as detected by contrast-enhanced cardiac magnetic resonance imaging, regardless of the presence or absence of angiographically detectable distal embolisation11.
Although many experts believe that the coronary microcirculation is the next frontier in reperfusion therapy for STEMI12,13, the strong impact of thrombus burden in the prediction of post-procedural microvascular dysfunction after PPCI in STEMI patients as observed in the study by De Maria et al pinpoints distal embolisation of atherothrombotic material as a major target for further studies, and for development of new interventional techniques and pharmacologic strategies. Unfortunately, the “egg of Columbus” approach with manual aspiration of thrombus through rigid large bore catheters did not translate into a better clinical outcome when routinely performed in a very large number of STEMI patients and was associated with a higher risk of stroke14,15. Recently, it was shown that thrombus aspiration during PPCI resulted in a worsening of the ST-segment elevation in 20% of the patients, possibly by causing embolisation of atherothrombotic fragments into the microcirculation16.
In conclusion, although PPCI is very effective in recanalising the epicardial coronary artery, prevention and treatment of microvascular obstruction remains a major unmet need in one third of STEMI patients. Selective use of more refined thrombectomy catheters (e.g., as used in the context of stroke) in patients with a large thrombus burden may possibly lead to a reduction of the incidence of microvascular obstruction caused by distal embolisation. Whether more refined, safe and effective thrombectomy catheters will be efficacious in improving prognosis further is unpredictable, but it will certainly be possible to evaluate any new strategy accurately for the prevention of microvascular obstruction in STEMI in small study groups by periprocedural IMR measurements, as used in the present study.
Funding
The Department of Cardiology, Antwerp University Hospital and University of Antwerp, has received research grants from Medtronic and Abbott.
Conflict of interest statement
The authors have no conflicts of interest to declare.

