
Abstract
Aims: The aim of this study was to determine the prognostic impact of pre- and post-PCI TIMI flow grade and TIMI myocardial perfusion grade (TMPG) in a well-defined group of patients with cardiogenic shock due to acute myocardial infarction.
Methods and results: Patients with infarct-related cardiogenic shock randomised into the CULPRIT-SHOCK trial were included in the angiographic predictor analysis whenever their TIMI flow grade or TMPG was available in the core lab database (96.9% of cases). A multivariable logistic regression analysis, adjusted on non-angiographic covariates, was performed to investigate whether TIMI flow grade or TMPG was independently associated with all-cause mortality or renal replacement therapy up to one year. Pre-PCI TIMI flow grade and TMPG did not impact on mortality. When analysed in separate multivariable models, post-PCI TIMI 3 flow and TMPG grade 3 were both significantly associated with reduced risk of 30-day mortality: aOR 0.61 (95% CI: 0.38-0.97, p=0.037) and 0.46 (95% CI: 0.29-0.72, p<0.001), respectively. When considered in the same multivariable model, only TMPG was significantly associated with 30-day mortality (aOR 0.38 [0.20-0.71], p=0.002), the 30-day composite of all-cause mortality and renal replacement therapy (aOR 0.34 [0.18-0.66], p=0.001) and mortality at one-year follow-up (aOR 0.46 [0.24-0.88], p=0.02).
Conclusions: Post-PCI TIMI flow grade and TMPG are associated with mortality after PCI. TMPG is a better discriminator, supporting microcirculation rather than epicardial reperfusion for prognosis estimation.
Introduction
Percutaneous coronary intervention (PCI) reduces mortality in patients presenting with acute myocardial infarction (MI)1,2 irrespective of the presence of cardiogenic shock3. However, the estimation of the efficacy of percutaneous revascularisation can be further stratified by angiographic measures such as Thrombolysis In Myocardial Infarction (TIMI) flow grade, TIMI myocardial perfusion grade (TMPG) or myocardial blush grade as well as by TIMI frame count (TFC). Indeed, TIMI flow grade, TMPG and TFC have been proven to be associated with mortality in patients with MI predominantly without cardiogenic shock and have therefore been used to define the success of revascularisation4,5,6,7. Nevertheless, evidence concerning these parameters in patients with cardiogenic shock is scarce8,9, and systemic microcirculation and macrocirculation might be more relevant than myocardial reperfusion. The CULPRIT-SHOCK trial demonstrated that percutaneous revascularisation of the culprit coronary artery only was superior to an immediate multivessel PCI in patients with acute MI with multiple vessel coronary artery disease (CAD) and cardiogenic shock10.
We sought to determine whether angiographic revascularisation indicators (TIMI flow grade, TMPG), blindly evaluated in an angiographic core laboratory, are associated with outcome after PCI in a well-defined cohort of cardiogenic shock patients with acute MI and multivessel CAD.
Methods
STUDY POPULATION
The CULPRIT-SHOCK randomised, open-label, multicentre trial demonstrated the superiority of culprit lesion-only PCI with possible staged revascularisation over an immediate multivessel PCI strategy in patients with cardiogenic shock related to MI, regarding all-cause mortality or renal replacement therapy (RRT) at 30 days and one year10,11. Patients included in the CULPRIT-SHOCK trial had a core lab angiographic blinded evaluation of their coronary angiograms. We analysed all patients with reported data on pre-PCI TIMI flow grade or TMPG and considered pre- and post-PCI TIMI flow grade and TMPG of the culprit artery only.
ANGIOGRAPHIC CORE LABORATORY
The ACTION (Allies in Cardiovascular Trials, Initiatives and Organized Networks) angiographic core laboratory (Institut de Cardiologie, Pitié-Salpêtrière Hospital) was blinded to patient characteristics, outcomes and randomisation group. For each patient, two blinded readers adjudicated the angiographic parameters (TIMI flow grade, TMPG) of the culprit artery, as previously described12.
TIMI flow grade and TMPG are complementary angiographic measurements representative of coronary circulation. TIMI flow grade evaluates the quality of coronary flow in epicardial vessels by measuring coronary artery clearance of radiographic dye. TMPG evaluates the quality of coronary flow in the myocardial microvasculature. TIMI flow grade and TMPG were recorded in accordance with their original definitions4,12,13 (Supplementary Table 1, Supplementary Figure 1).
All data were entered into a dedicated computerised database. In case of disagreement between readers, a third and eventually a fourth independent reader were requested to reach a consensus. For the purposes of this study, angiographic parameters were dichotomised into TIMI flow grade <3 versus TIMI flow grade 3 and TMPG <3 versus TMPG 3.
OBJECTIVE AND OUTCOMES
We hypothesised that the angiographic measures used in clinical practice for estimation of the efficacy of percutaneous revascularisation are associated with prognosis after PCI for acute MI complicated by cardiogenic shock. In addition, we hypothesised that their relative importance would differ.
The objective was to determine whether TIMI flow grade and TMPG (3 versus <3 for both parameters) before and after PCI are associated with short- and long-term outcomes. Outcomes of interest for this substudy were all-cause mortality at 30 days, all-cause death or renal replacement therapy at 30 days, and all-cause mortality at one-year follow-up. Recurrent MI and rehospitalisation for heart failure at 30 days were also considered.
STATISTICAL ANALYSIS
Continuous variables are reported as mean±standard deviation (SD). Categorical variables are reported as number (%). To investigate the association between angiographic parameters and outcomes, we evaluated TIMI flow grade and TMPG (3 versus <3 for both parameters) as separate and dependent variables in different multivariable models. Multivariate logistic regression models were used to evaluate the independent association between TIMI flow grade or TMPG and outcomes. In each model, TIMI flow grade or TMPG was adjusted for baseline clinical and procedural characteristics significantly associated with outcomes on univariate analysis (p<0.2). To investigate which angiographic parameter would be the most clinically relevant, we also built a multivariable model including both TIMI flow grade and TMPG. The list of covariables is as follows: age, gender, body mass index, current smoking, hypercholesterolaemia, diabetes mellitus, chronic renal failure, previous PCI, previous stroke, previous coronary artery bypass grafting (CABG), arterial lactate >2 mmol/l, fibrinolysis before randomisation, left main or left anterior descending (LAD) culprit artery, ≥1 chronic total occlusion, femoral access, stent implantation in culprit artery, randomisation group, mechanical circulatory support, mechanical ventilation, and catecholamine therapy.
The main analyses were performed entering randomisation group as a covariable. However, in the CULPRIT-SHOCK trial the crossover rate was approximatively 10%. Hence, sensitivity analyses were performed entering the type of revascularisation procedure (culprit only versus multiple vessel PCI) instead of randomisation group as a covariable in the multivariable analysis (“as-treated” analysis) to investigate the possible impact of crossovers and type of revascularisation on outcomes.
Results are reported as adjusted odds ratios (aOR) with their 95% confidence intervals (95% CI). A p-value <0.05 was considered significant. All statistical analyses were performed with SAS statistical software, release 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Out of the 686 patients included in the CULPRIT-SHOCK trial, 665 (96.9%) were included in the angiographic substudy. Mean patient age was 68.5±11.4 years, one third had diabetes mellitus and 24% were female. Two thirds of the patients presented with ST-segment elevation myocardial infarction (STEMI), most had triple-vessel disease (64%) and the LAD was the most frequent culprit artery (42%). Severe cardiogenic shock was common, as attested by catecholamine use in 90% of patients and mechanical circulatory support in 29% of patients. All-cause mortality was 47% and 53% at 30 days and one year, respectively (Table 1, Table 2).
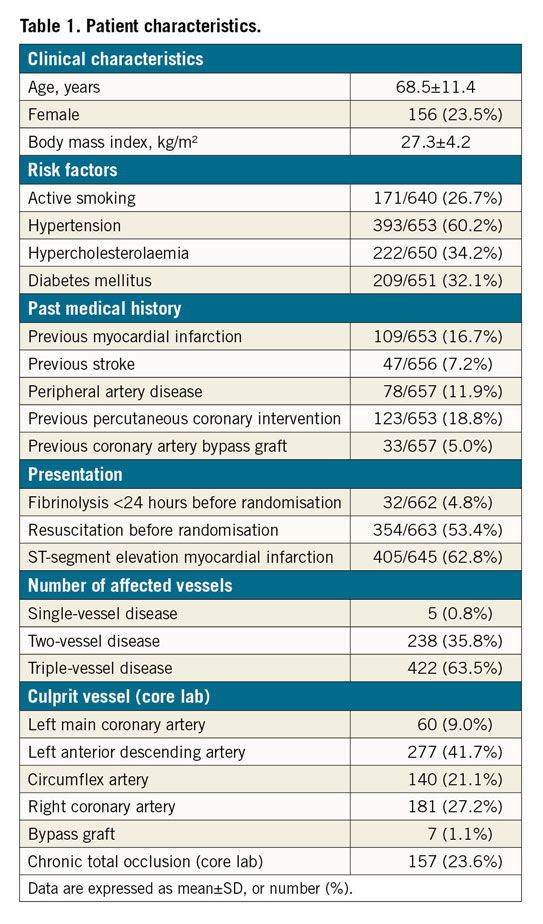
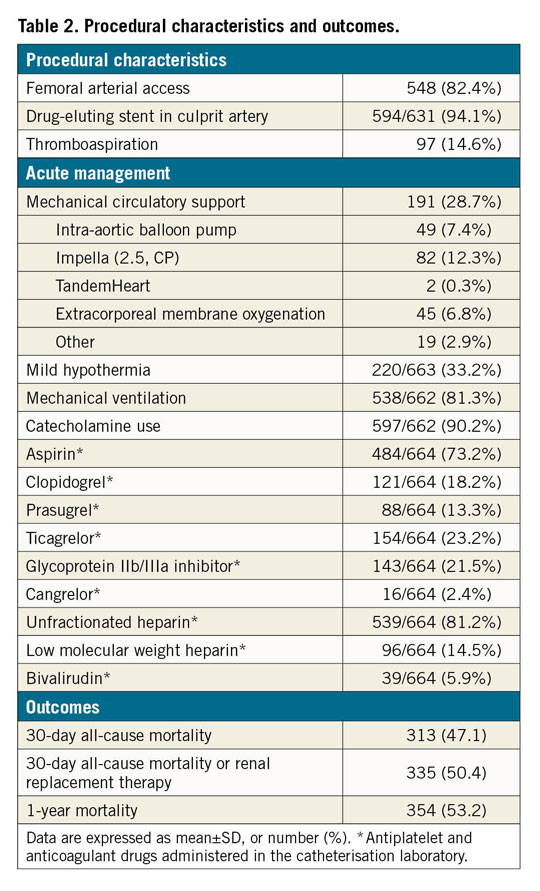
TIMI FLOW GRADE AND TMPG
As per the angiographic core lab, for the culprit artery TIMI flow grade and TMPG before PCI were available in 663 (99.7%) and 598 (89.9%) patients, whereas TIMI flow grade and TMPG post PCI were available in 639 (96.1%) and 504 (75.8%) patients, respectively. The incompleteness of core lab angiographic data is due mainly to the limited availability or inadequate quality of the angiographic films. Before PCI, the number of patients with TMPG 3 and TIMI flow grade <3 was 44 (19.4%), almost exclusively represented by patients with TIMI flow 2. Before PCI, the number of patients with TIMI flow grade 3 and TMPG <3 was 12 (3.2%). Before PCI, TIMI flow grade was 3 in 220 (33.2%) patients and TMPG was 3 in 228 (38.1%) patients. Post PCI, TIMI flow grade 3 was achieved in 499 (78.1%) patients and TMPG 3 in 320 (63.5%) patients. The comparison of effects of the different grades of post-PCI TIMI flow grade and TMPG on 30-day mortality, 30-day mortality or RRT, and one-year mortality displayed an apparently stepwise relationship (Table 3, Table 4, Figure 1-Figure 3). The multivariable analysis including both post-PCI TIMI flow grade and TMPG included 463, 449 and 464 patients for the endpoints 30-day mortality, 30-day mortality or RRT, and one-year mortality, respectively.

Figure 1. Proportions of 30-day all-cause mortality according to post-PCI TIMI flow grade or TMPG.
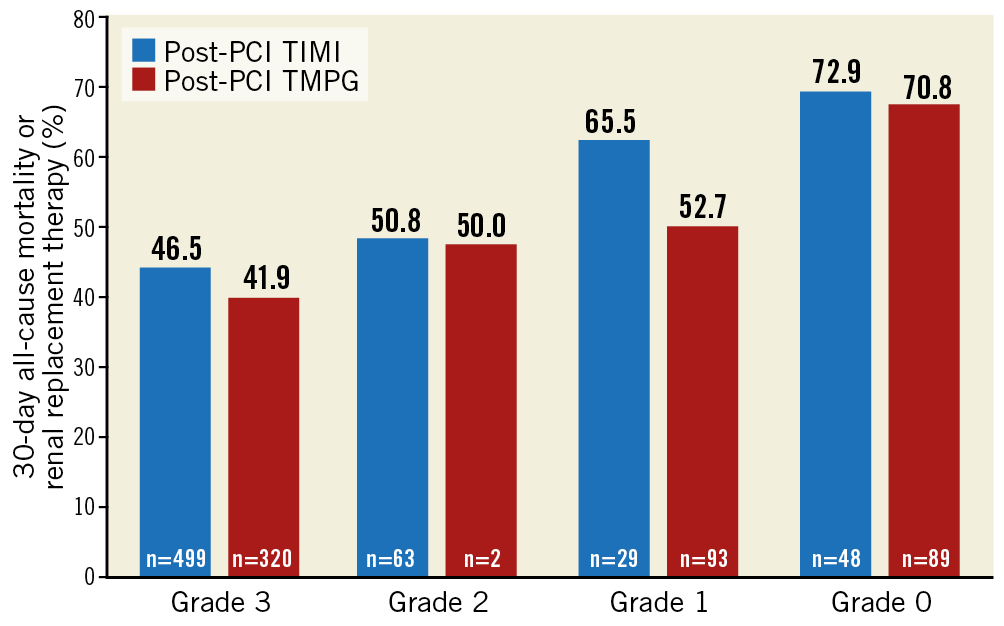
Figure 2. Proportions of 30-day all-cause-mortality and renal replacement therapy according to post-PCI TIMI flow grade or TMPG.
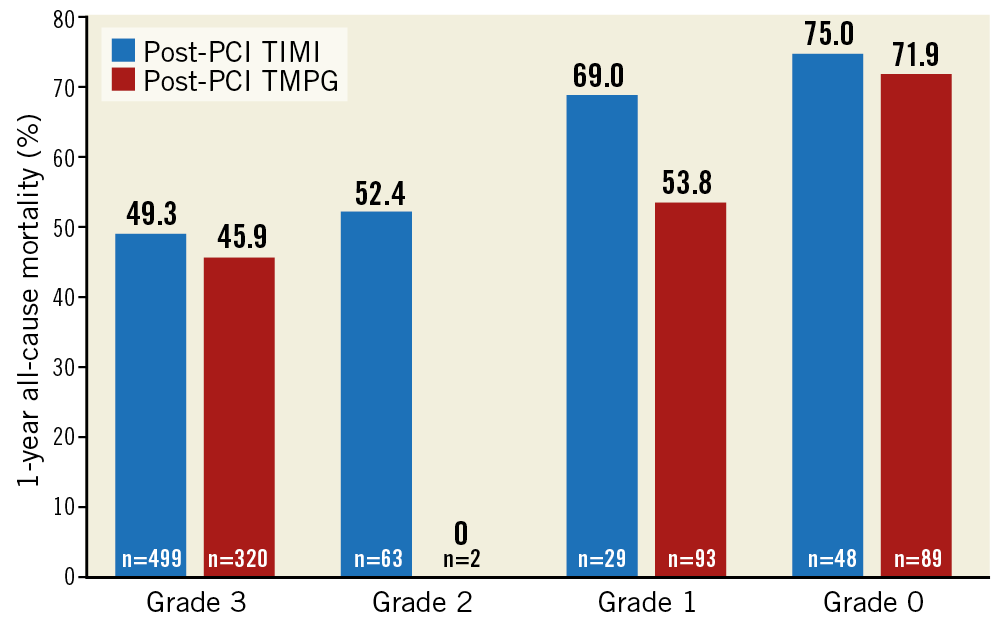
Figure 3. Proportions of one-year all-cause mortality according to post-PCI TIMI flow grade or TMPG.
ANGIOGRAPHIC PARAMETERS AND 30-DAY ALL-CAUSE MORTALITY
Neither TIMI flow grade nor TMPG pre-PCI was associated with 30-day mortality after univariate analysis (p=0.56 and 0.11, respectively). Both TIMI flow grade and TMPG (3 versus <3 for both parameters) in the culprit artery after PCI were associated with 30-day mortality in univariate analysis (p<0.001). TIMI flow 3 and TMPG 3 both remained significantly associated with lower risk of 30-day mortality after adjustment for other non-angiographic covariates in respective multivariable models, with an aOR of 0.61 (95% CI: 0.38-0.97), p=0.037 for TIMI flow grade, and aOR of 0.46 (95% CI: 0.29-0.72), p<0.001 for TMPG. When included in the same multivariable model, the statistical association of TIMI flow grade with 30-day mortality was offset (aOR 1.27 [95% CI: 0.62-2.64], p=0.51) by the effect of TMPG (aOR 0.38 [95% CI: 0.20-0.71], p=0.002), which remained significant (Figure 4, Supplementary Table 2).
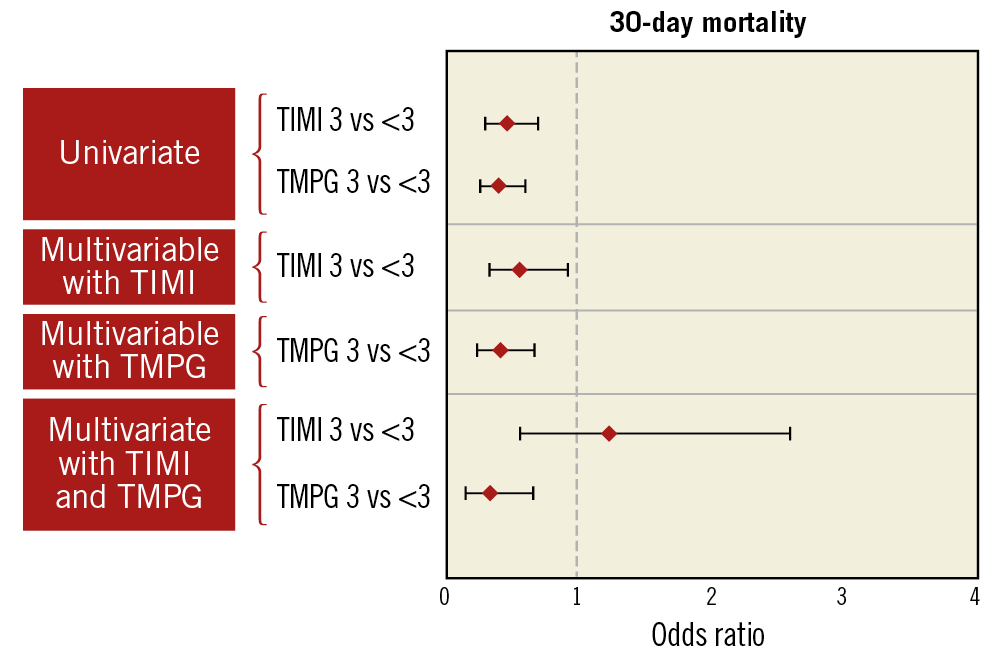
Figure 4. Univariate and multivariable analysis of association of post-PCI TIMI flow grade and TMPG with 30-day all-cause mortality.
ANGIOGRAPHIC PARAMETERS AND OTHER ENDPOINTS
Neither TIMI flow grade nor TMPG pre-PCI (3 versus <3 for both parameters) was associated with the composite endpoint of all-cause mortality or RRT at 30 days on univariate analysis (p=0.43 and 0.16, respectively). Both TIMI flow grade and TMPG in the culprit artery after PCI were associated with the composite endpoint of all-cause mortality or RRT at 30 days on univariate analysis. After adjustment for non-angiographic covariates, the association of TMPG remained significant with an aOR of 0.48 (95% CI: 0.30-0.77), p=0.002, while the association of TIMI flow grade did not (aOR 0.73 [95% CI: 0.45-1.18], p=0.20) when included in separate multivariable models. When included in the same multivariable model, TIMI flow grade was not associated with outcome (aOR 1.65 [95% CI: 0.76-3.56], p=0.21) while the association of TMPG remained significant (aOR 0.34 [95% CI: 0.18-0.66], p=0.001) (Figure 5, Supplementary Table 3).

Figure 5. Univariate and multivariable analysis of association of post-PCI TIMI flow grade and/or TMPG (separately then together) with 30-day all-cause-mortality and renal replacement therapy
Neither TIMI flow grade nor TMPG pre-PCI (3 versus <3) was associated with one-year mortality on univariate analysis (p=0.98 and 0.27, respectively). Both TIMI flow grade and TMPG in the culprit artery after PCI were associated with one-year all-cause mortality on univariate analysis (p=0.003 and p<0.001, respectively). After adjustment for non-angiographic covariates in separate multivariable models, the association with one-year mortality of both TIMI flow grade and TMPG after PCI was found to be non-significant (p=0.41 for TIMI flow grade and p=0.065 for TMPG). When included in the same multivariable model, TIMI flow grade was not associated with one-year all-cause mortality (p=0.21), while TMPG was significantly associated with the outcome (aOR 0.46 [95% CI: 0.24-0.89], p=0.02) (Figure 6, Supplementary Table 4).
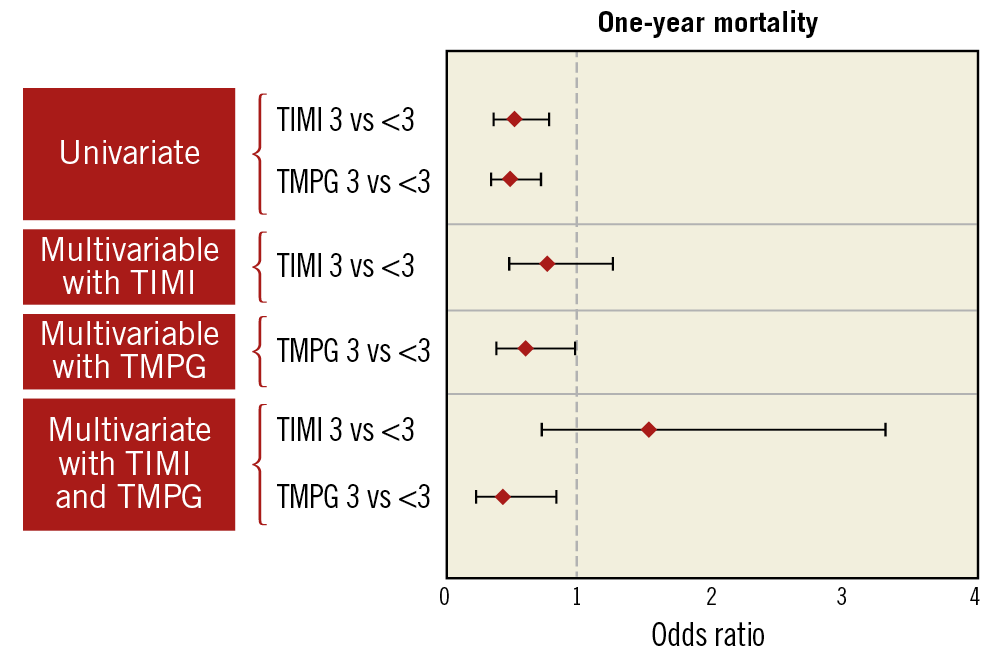
Figure 6. Univariate and multivariable analysis of association of post-PCI TIMI flow grade and/or TMPG (separately then together) with one-year all-cause mortality.
Interestingly, 70 (18.3%) patients with post-PCI TIMI flow grade 3 had TMPG <3, of whom 8 (2.1%) had TMPG 0. Mortality in patients with post-PCI TIMI flow 3 and TMPG 0 was 50% at 30 days and one year. No significant interactions between the PCI strategies (i.e., culprit lesion-only or multivessel PCI) and each angiographic parameter were observed for any outcomes. No difference was noted between characteristics of patients analysed versus those not analysed by the multivariable models which included both TIMI flow grade and TMPG as covariates. No univariate association was found between TIMI flow grade or TMPG and rehospitalisation for heart failure or recurrent MI. The sensitivity analyses were concordant with the main analyses (Supplementary Table 5). No association was found between TIMI flow grade and TMPG (3 versus <3 for both) and RRT (p=0.99 and p=0.12, respectively), recurrent MI (p=0.13 and p=0.99, respectively) or rehospitalisation for heart failure (p=0.13 and p=0.36, respectively) on univariate analysis.
Discussion
In this study we report that both TIMI flow grade and TMPG after PCI are associated with all-cause mortality at 30 days in patients presenting with acute MI, multivessel disease and cardiogenic shock. However, when the effect of both angiographic parameters is evaluated jointly in the same model, only TMPG remained associated with mortality at 30 days as well as one year after PCI.
Our results echo those of Mehta et al, who found that post-PCI TIMI flow grade in the culprit artery had a graded inverse relationship with adjusted risk of in-hospital mortality in patients with cardiogenic shock undergoing PCI. Indeed, they found that the OR (95% CI) for TIMI flow grades 0/1 and 2 was 5.47 (95% CI: 4.13 to 7.24) and 2.63 (95% CI: 2.02 to 3.42) compared with TIMI flow grade 3, respectively. However, contrary to Mehta et al, our analysis is more robust because it is based on data extracted from the CULPRIT-SHOCK randomised controlled trial using an angiographic core lab and with rigorous follow-up. Also, the prognostic effect beyond the in-hospital stay and the relationship with TMPG were not previously evaluated by Mehta et al8.
The strong association of TMPG with the vital status suggests that myocardial microcirculation provides better information than epicardial coronary flow on the efficacy of PCI reperfusion and on the prognosis of these patients admitted in cardiogenic shock. Perhaps unsurprising given previous validation of TMPG as a prognostic marker and infarct size correlate after thrombolysis as well as PCI in MI, our results provide insight for severely ill patients with cardiogenic shock in a contemporary cohort of acute MI patients treated with PCI. The higher prognostic association of TMPG compared to TIMI flow grade after PCI, as attested by our multivariable models, has not been previously described4,5,6,7,14,15. Approximately one fifth of patients with optimal TIMI flow after PCI in the culprit artery had TMPG <3. Although myocardial microcirculation function can be appreciated angiographically with the TMPG, it can also be estimated through ST-segment elevation resolution in STEMI patients, which has been proposed to have a closer relationship to prognosis than TIMI flow16. Operators should consider aiming at optimising the microvascular angiographic result during PCI procedures, and not focus only on the post-procedure TIMI flow grade.
Coronary revascularisation reduces mortality and successful PCI yields better survival in patients with ischaemic cardiogenic shock3,17. However, abnormal TIMI flow grade or TMPG at the end of a PCI procedure is associated with worse prognosis. This phenomenon corresponds to slow reflow when partial angiographic filling of vessels is observed, or to no reflow when no flow circulates in the coronary artery. Its mechanism is thought to be associated with atherothrombotic micro-embolisation, reperfusion injury, and spasm. Slow/no reflow has been reported in 10% up to 25% of cases and is associated with high thrombus burden and late reperfusion on admission18,19,20. Intravenous adenosine, calcium channel blockers, and glycoprotein IIb/IIIa inhibitors have been proposed to improve coronary perfusion and reduce infarct size, although the clinical benefit remains unproven21,22,23,24,25. Reversing no/slow reflow could reduce myocardial rhythmic susceptibility and size of the myocardial scar, factors associated with early and late mortality. New effective strategies of myocardial protection during the acute phase of MI and of coronary reperfusion are seriously needed.
Limitations
TIMI frame count (TFC) is another angiographic parameter, along with TIMI flow grade and TMPG, that has been reported to be associated with prognosis after PCI12. TFC was unreliable in the CULPRIT-SHOCK core lab database due to insufficient quality of angiographic cines for this parameter. Myocardial blush grade (MBG) is another angiographic surrogate representing coronary microcirculation26. Both TMPG and MBG were reported to be correlates of mortality after revascularisation. While MBG can be favoured by clinicians for its simplicity, it was not recorded in the CULPRIT-SHOCK trial. Missing data for angiographic predictors partially impaired the multivariable analyses that included both post-PCI TIMI flow grade and TMPG, with approximately 70% of patients in the analysed population. However, no difference was noted between analysed and non-analysed patients in the multivariable analysis. Angiographic indices were recorded twice (pre and post PCI) by the core laboratory, hence the impact of the different therapeutic interventions (e.g., variations in blood pressure, revascularisation of each vessel) could not be appreciated in a repeated fashion. Developing new strategies to improve microvascular dysfunction after MI is warranted; however, this study does not provide new leads for it.
Conclusions
The angiographic parameters post-PCI TIMI flow grade and TMPG are associated with mortality after PCI in patients with acute MI and cardiogenic shock. TMPG appears to be a better discriminator than TIMI flow grade to evaluate the success of PCI reperfusion in these cardiogenic shock patients.
|
Impact on daily practice After percutaneous coronary intervention, only TIMI myocardial perfusion grade was independently significantly associated with all-cause mortality at 30 days and one year after adjustment for clinical covariates and TIMI flow grade in this angiographic substudy of the CULPRIT-SHOCK trial. This study provides additional data to support the notion that microcirculation matters more than the epicardial reperfusion after percutaneous coronary intervention in cardiogenic shock patients. |
Appendix. Study collaborators
Benjamin Bertin, MD; Delphine Brugier, MD; Nicolas Vignolles, MD; Georges Hage, MD; Mohamad El Kasty, MD; Mathieu Kerneis, MD; Sorbonne Université, ACTION Study Group, INSERM UMRS_1166, Institut de cardiologie (AP-HP), Paris, France. Kurt Huber, MD; Wilhelminenspital Kardiologie-Wien, Vienna, Austria. Marko Noc, MD; University Medical Centre Ljubljana, Ljubljana, Slovenia. Marcus Sandri, MD; Heart Center Leipzig at University of Leipzig and Leipzig Heart Institute, Leipzig, Germany. Georg Fuernau, MD; University Heart Center Luebeck, Luebeck, Germany.
Funding
The CULPRIT-SHOCK trial was supported by a grant agreement (602202) from the European Union Seventh Framework Program and by the German Heart Research Foundation and the German Cardiac Society.
Conflict of interest statement
P. Overtchouk has received research grants from the Federation Française de Cardiologie and the European Society of Cardiology. G. Montalescot has received research grants from Abbott, Amgen, Actelion, American College of Cardiology Foundation, AstraZeneca, Axis-Santé, Bayer, Boston Scientific, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, China Heart House, Daiichi Sankyo, Idorsia, Elsevier, Europa, Fédération Française de Cardiologie, ICAN, Lead-Up, Medtronic, Menarini, MSD, Novo-Nordisk, Partners, Pfizer, Quantum Genomics, Sanofi, Servier, and WebMD. H. Thiele reports grants from the European Union, the German Cardiac Society, and the Deutsche Stiftung für Herzforschung. U. Zeymer reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Novartis, Sanofi, MSD, The Medicines Company, Pfizer, Daiichi Sankyo, Eli Lilly, and Abiomed. M. Kerneis has received research grants from Institut Servier and Fédération Française de Cardiologie, as well as lecture fees from Sanofi, Bayer and Servier. J. Silvain has received research grants, honoraria or travel support from Amgen, Algorythm, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead Science, Bristol-Myers Squibb and Sanofi-Aventis. J.P. Collet has received research grants from AstraZeneca, Bayer, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Fédération Française de Cardiologie, Lead-Up, Medtronic, MSD, Sanofi-Aventis, and WebMD. E. Vicaut reports receiving personal fees from Eli Lilly; consultancy for Pfizer, Sanofi, LFB, Abbott, Fresenius, Medtronic, and Hexacath; being a member of the data safety monitoring board for CERC, and receiving lecture fees from Novartis, and grants from Boehringer and Sanofi. M. Zeitouni has received research grants from Federation Française de Cardiologie and Institut Servier. M. Noc reports personal fees from ZOLL Circulation, and Maquet Gettinge, and grants from AstraZeneca, outside the submitted work. The other authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

