
In this edition of EuroIntervention there are two papers addressing different aspects of the use of the index of microcirculatory resistance (IMR) in patients with stable ischaemic heart disease (SIHD) and acute ST-elevation myocardial infarction (STEMI).
The group from Oxford in the UK in collaboration with colleagues from Rome, Italy, has previously reported that a novel score called ATI predicted elevated IMR (>40) after stenting in patients undergoing primary percutaneous coronary intervention for STEMI1. The score incorporates age (>50 years), angiographically assessed thrombus burden (grades 0-5) and IMR, measured in the infarct-related artery after aspiration/balloon angioplasty has restored at least TIMI 2 flow but, importantly, before stent implantation. Within the ATI score, both thrombus burden and IMR are weighted, with IMR <40 scoring 1, IMR 40-100 scoring 2 and IMR >100 scoring 3. When IMR exceeds 100, there is usually angiographically obvious slow flow with very prolonged hyperaemic transit times which can be quite difficult to document as the system times out before registering the temperature curves. In the original validation of the ATI score, age >50 years only predicted post-stenting IMR >40 in univariable analysis but was retained in the score anyway. In the multivariable analysis, the odds ratio for thrombus score was 2.82 compared to only 1.03 for pre-stenting IMR >40, suggesting that the former parameter may be most predictive. Nevertheless, some patients with thrombus scores <4 do generate a high ATI score based on pre-stenting IMR >40.
In the current study, the same investigators report that the ATI score also predicts final infarct size and the degree of microvascular obstruction, both assessed using cardiac magnetic resonance imaging (CMR) and both expressed as a percentage of left ventricular mass. An elevated ATI score also correlated strongly with the incidence of myocardial haemorrhage (microvascular destruction) and salvage index2.
These findings are consistent with multiple previous reports of the ability of post-stenting IMR >40 to predict both CMR-derived parameters of the extent and pathological features of myocardial infarction as well as long-term clinical outcomes such as death and hospitalisation for heart failure3,4.
Finding out that there is physiological evidence of microvascular obstruction (IMR >40) after stenting in a patient with a STEMI is of value in terms of predicting outcome; however, the window for therapeutic intervention may already have been missed. Finding this out before stenting in conjunction with a high thrombus score should cause one to pause for at least a moment. What is going to happen here when I place a stent? Will the flow get better or worse? In most cases, even with TIMI 3 flow after aspiration/balloon angioplasty, there will be a residual stenosis in the epicardial artery. Fixing this by stenting is what we routinely do and may indeed improve flow. Nevertheless, when you have information in the lab indicating a high probability that immediate stent implantation will result in a larger final infarction, one could make a compelling argument for considering some modification of the therapeutic strategy. One option is delayed stenting, with additional time being given for pharmacological therapy to reduce the residual thrombus burden and open up the microcirculation. The DANAMI-DEFER-3 study, which explored this strategy, was negative but it recruited an all-comers population and the delay to stenting may have been too long. The investigators did not measure either IMR or angiographic thrombus burden but, in the CMR substudy, there was a strong signal for a reduction in infarct size in patients with a stent length >24 mm, a metric that could be considered a surrogate for atherothrombotic burden5. The clinical utility of a modified ATI score incorporating lesion (stent) length could be tested by randomising patients with STEMI and high scores to immediate versus delayed stenting. As Professor Banning has also previously proposed, such a trial would have the built-in added bonus of identifying very low-risk patients who could be directed towards a very rapid discharge pathway.
As discussed above, IMR in the infarct-related artery of patients with STEMI tells us something about how much viable myocardium will be left when the dust has settled. In a second paper in this edition of EuroIntervention, a multinational group from Spain, the Netherlands and the UK reports on the relationship between IMR and perfused myocardial mass in a cohort of patients with SIHD6.
The authors used three different angiographically derived measures of perfused myocardial mass rather than a CMR- or positron emission tomography (PET)-based methodology and so cannot specifically comment on viability. However, patients known to have had prior infarction in the territory of the vessels studied were excluded and the average IMR values were similar to previously reported populations with SIHD, so it is reasonable to assume that these vessels were supplying mostly viable myocardium. In a population of 123 coronary arteries in 102 patients with a clinical indication for pressure wire assessment, there was a weak but statistically significant curvilinear inverse correlation between myocardial mass and IMR (r2=0.16; p<0.001). Independent predictors of IMR included myocardial mass, aortic pressure, minimum lumen diameter and age6. Interestingly, age was not a predictor of IMR in a previously published larger observational study7 but IMR was higher in the right coronary artery which in general supplies less myocardium than the left anterior descending artery. This observation fits with the data of Echavarria-Pinto et al6.
In theory, resistance to flow in normal branching epicardial coronary arteries should increase from proximal to distal, whilst pressure is maintained and flow decreases. As such, some of the variation in IMR measurements observed clinically may be explained by the position of the sensor in the artery at the time the data are collected and thereby the mass of perfused myocardium being interrogated. In truly normal vessels, during hyperaemia, thermodilution-derived transit times (one of the two components of IMR) correlate very well with absolute flow measurements when the sensor is placed at least 6 cm distal to the coronary ostium and there is minimal change with more distal sensor positions8. However, the patients in the current study had coronary atherosclerosis and lesions angiographically severe enough to justify measurements of fractional flow reserve (FFR) as witnessed by a median FFR value of 0.83. In other words, pressure was not maintained from proximal to distal in these diseased vessels. The investigators did not have coronary wedge pressure measurements (Pw) to correct IMR for this epicardial disease and so they used FFR and Yong’s formula9. This did not seem to alter the weak relationship between myocardial mass and IMR. However, the correction was only employed for vessels with an FFR <0.75 and the correlation between IMR corrected using Yong’s formula rather than Pw, although good, is not exact (r2=0.87). No relationship could be demonstrated between coronary flow reserve (CFR) and myocardial mass and the authors suggest that, as a ratio of resting to hyperaemic flow, CFR “intrinsically corrects” for the mass of perfused myocardium. This finding may cast some light on the slightly puzzling combination of vessels with high IMR but apparently preserved CFR. Are these simply vessels with normal coronary function but small perfusion territories? Alternatively, it is equally plausible that CFR, which is not a specific index of microvascular function, has been reduced in these vessels by the influence of often angiographically undetectable epicardial disease. Ultimately, we agree with the authors that, even if it is real, the rather small influence of myocardial mass on IMR is insufficient to negate its utility as a metric of microvascular function. However, as with the role of IMR in patients with STEMI, we need randomised controlled trials in patients with SIHD, or indeed isolated coronary microvascular dysfunction in which IMR is used to select patients and/or guide therapy, before it will assume a major role in routine practice. Meantime, we offer a flow chart of how IMR (and the ATI score) could currently be incorporated into clinical practice in these two distinct patient populations (Figure 1).
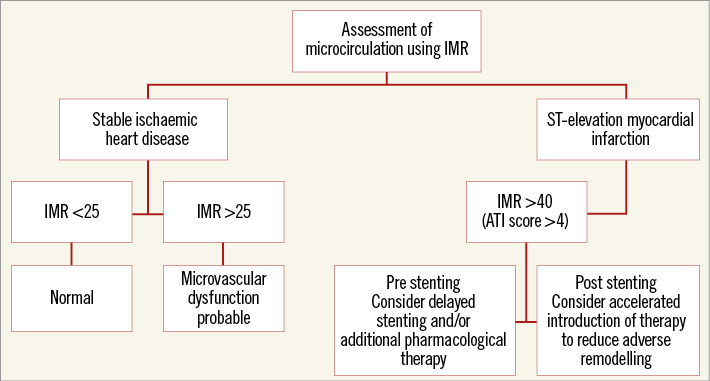
Figure 1. A schema of how IMR measurements could be incorporated into clinical practice.
Conflict of interest statement
The authors have no conflicts of interest to declare.

