
Abstract
Aims: The age-thrombus burden-index of microcirculatory resistance (ATI) score is a diagnostic tool able to predict suboptimal myocardial reperfusion before stenting, in patients with ST-elevation myocardial infarction (STEMI). We aimed to validate the ATI score against cardiac magnetic resonance imaging (cMRI).
Methods and results: The ATI score was calculated prospectively in 80 STEMI patients. cMRI was performed within 48 hours in all patients and in 50 patients at six-month follow-up to assess the extent of infarct size (IS%) and microvascular obstruction (MVO%). The ATI score was calculated using age (>50=1 point), pre-stenting index of microcirculatory resistance (IMR) (>40 and <100=1 point; ≥100=2 points) and angiographic thrombus score (4=1 point; 5=3 points). ATI score was closely related to final IS% (ATI0-1: 16.5% [8.7-22.9], ATI2-3: 28.5% [15.5-35.0], ATI4-5-6: 35.5% [22.2-44.4], p=0.001) and with MVO% (ATI0-1: 0.0% [0.0-0.9], ATI2-3: 0.7% [0.0-2.5] and ATI4-5-6: 4.1% [1.2-10.7], p<0.001). Furthermore, ATI score predicted final IS% at six-month follow-up (ATI0-1: 12.7% [4.1-18.0], ATI2-3: 16.30% [8.0-24.7], ATI4-5-6: 29.5% [19.9-49.5], p=0.02).
Conclusions: The ATI score performed prior to stenting in patients with STEMI can predict the likelihood of MVO% and IS% both acutely and at six-month follow-up cMRI.
Abbreviations
ATI: age-thrombus burden-index of microcirculatory resistance
AUC: area under the curve
cMRI: cardiac magnetic resonance imaging
DES: drug-eluting stent
DS%: percentage diameter stenosis
EDV: end-diastolic volume
GP IIb/IIIa: glycoprotein IIb/IIIa
IMR: index of microcirculatory resistance
IQR: interquartile range
IS: infarct size
IS%: percentage infarct size
LAD: left anterior descending
LCx: left circumflex
LV: left ventricle
MBG: myocardial blush grade
MLA: minimal lumen area
MVO: microvascular obstruction
MVO%: percentage microvascular obstruction
pPCI: primary percutaneous coronary intervention
RCA: right coronary artery
STEMI: ST-elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
TS: thrombus score
T2W: T2 weighted
∑STR: ST resolution
2D-QCA: two-dimensional quantitative coronary angiography
Introduction
Prompt revascularisation with primary percutaneous coronary intervention (pPCI) and stenting of the culprit vessel is the current gold standard treatment for patients with ST-elevation myocardial infarction (STEMI)1. However, suboptimal myocardial reperfusion occurring as a consequence of coronary microvascular injury is observed in a proportion of patients and is known to be associated with adverse clinical outcomes2.
The ability to identify STEMI patients at the highest risk of post-procedural microvascular injury before stenting is potentially valuable, as it facilitates the triage of additional and/or alternative therapeutic strategies to the conventional approach. We have proposed a risk stratification tool based on the combination of clinical (age), angiographic (thrombus score [TS]) and coronary physiology-derived (index of microcirculatory resistance [IMR]) parameters3 for initial prediction of a surrogate endpoint of post-pPCI myocardial reperfusion represented by the final index of microcirculatory resistance (IMR) after stent implantation. The age-thrombus burden-index of microcirculatory resistance (ATI) score has been validated in four distinct cohorts of patients, providing good accuracy to predict a post-procedural IMR greater than 403, a threshold which has previously been shown to be associated with adverse prognosis after STEMI4.
In the current study, we aimed to assess the ability of the ATI score to predict the actual infarct size and the extent of microvascular injury assessed by cardiac magnetic resonance imaging (cMRI) in STEMI patients.
Methods
Patients with STEMI admitted to the Oxford Heart Centre for pPCI were prospectively considered for enrolment (Figure 1). Symptom duration >12 hrs, presence of severe haemodynamic instability, severe left main disease, contraindications to adenosine infusion, plain old balloon angioplasty without stent implantation and general contraindication to cMRI were all exclusion criteria for the study. This study population was recruited as part of the Ox-AMI (Oxford Acute Myocardial Infarction) study (REC number 10/H0408/24). The study protocol was approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki.

Figure 1. Study flow chart.
pPCI was performed according to international guidelines and decisions about direct stenting technique, thrombectomy and glycoprotein (GP) IIb/IIIa adoption were all left to operator discretion. All patients were loaded with double antiplatelet therapy. Weight-adjusted unfractionated heparin or bivalirudin was adopted as antithrombotic regimen.
Angiographic TS was graded from 0 to 5 after the passage of the guidewire, as previously described5.
In all patients, IMR was measured immediately before (i.e., after thrombus aspiration and/or predilation, whenever performed) and after stenting as previously described6.
ATI SCORE
The ATI score was calculated in all patients before stenting (as previously described) by combining age (1 point for age >50), TS (1 point for TS=4, 3 points for TS=5) and pre-stenting IMR (1 point for IMR >40 and <100; 2 points for IMR ≥100). The final ATI score was obtained by summing the points given to each variable, resulting in a score ranging from zero to six. Details about the derivation and validation of the ATI score have been described previously3.
As previously reported, an ATI score ≥4 is strongly associated with a post-procedural IMR >40, whereas patients with an ATI score <2 represent a low-risk subgroup with none of these patients in our previous series having a final IMR >40. Patients with an ATI score of 2 or 3 represent an intermediate-risk group with a reported rate of post-procedural IMR >40 of 30%3.
CARDIAC MAGNETIC RESONANCE IMAGING
cMRI was performed using a 3.0 Tesla MR scanner (either MAGNETOM TIM Trio or MAGNETOM Verio; Siemens Healthcare, Erlangen, Germany) within 48 hours after pPCI and at six-month follow-up. Sequence acquisition and image analysis were performed as previously described7.
Quantification of area at risk and infarct size (IS) mass was performed on T1 and late gadolinium enhancement, respectively, and expressed as percentage of left ventricle (LV) mass8,9. Post-pPCI myocardial salvage index was measured as:
myocardial salvage index=[(area at risk–infarct size)/area at risk]*1008.
Furthermore, microvascular obstruction (MVO) was quantified by manual delineation of the hypointense areas within the infarct region and calculated as a percentage fraction of the LV (MVO%)10,11. The presence of haemorrhage was assessed visually on T2* maps and/or T2W as hypointense core inside the hyperenhancement region12.
Percentage change in IS at six months was assessed as:
IS shrinkage=[(ISpostPPCI–IS6months)/ISpostPPCI]*10013.
Variation of end-diastolic volume (EDV) between baseline and follow-up was assessed as:
EDV variation%=[(EDVpostPPCI–EDV6months)/EDVpostPPCI]*100.
A negative value of EDV variation% with a cut-off ≤–20% was considered diagnostic for adverse negative remodelling14.
STATISTICAL ANALYSIS
After assessing normal distribution by Shapiro-Wilk test, all variables were expressed as mean and (±) standard deviation or as median accompanied by interquartile range (IQR), as appropriate. In order to allow the use of parametric tests, base 10 logarithmic transformation – Log (x) or Log(x+k) with k being a constant in case of x presenting null or negative values – was applied. Frequency comparisons were made using the chi-square test or Fisher’s exact test, as appropriate, while continuous variables were compared by using a t-test or analysis of variance (ANOVA) with Scheffe’s post hoc comparisons, as appropriate. A t-test for paired samples was applied to assess change in IS% at six-month follow-up in the three ATI score subgroups.
Independent predictors of post-pPCI and six-month IS% were assessed by adopting a linear regression model after correcting for gender, diabetes, hypertension, hypercholesterolaemia, active smoking, ischaemic time, renal function, left anterior descending artery (LAD) as culprit vessel, Thrombolysis In Myocardial Infarction (TIMI) flow 0 at presentation, lesion length, percentage diameter stenosis, thrombectomy, bivalirudin adoption, use of GP IIb/IIIa inhibitors, predilation and post-dilation. ATI score and ATI score components, namely age, TS and pre-stenting IMR >40, were also included in the model.
Statistical analysis was performed using SPSS, Version 22.0 (IBM Corp., Armonk, NY, USA), and a p-value <0.05 was considered statistically significant.
Results
CLINICAL AND PROCEDURAL CHARACTERISTICS
Eighty patients presenting with STEMI underwent both coronary physiology and cMRI assessment and were then included in the current study, with 50 patients completing six-month follow-up with cMRI (Figure 1). The overall sample size consisted of two populations of STEMI patients recruited within the Ox-AMI study during two different time periods. The first 20 patients were recruited from January 2011 to September 2011, while the remaining 60 were recruited between May 2015 and September 2016. Notably, only 20 patients of the first cohort were included in the initial validation of the ATI score for prediction of final IMR >403.
Clinical and procedural characteristics are summarised in Table 1 and Table 2, and stratified according to the three classes of risk derived from the ATI score.
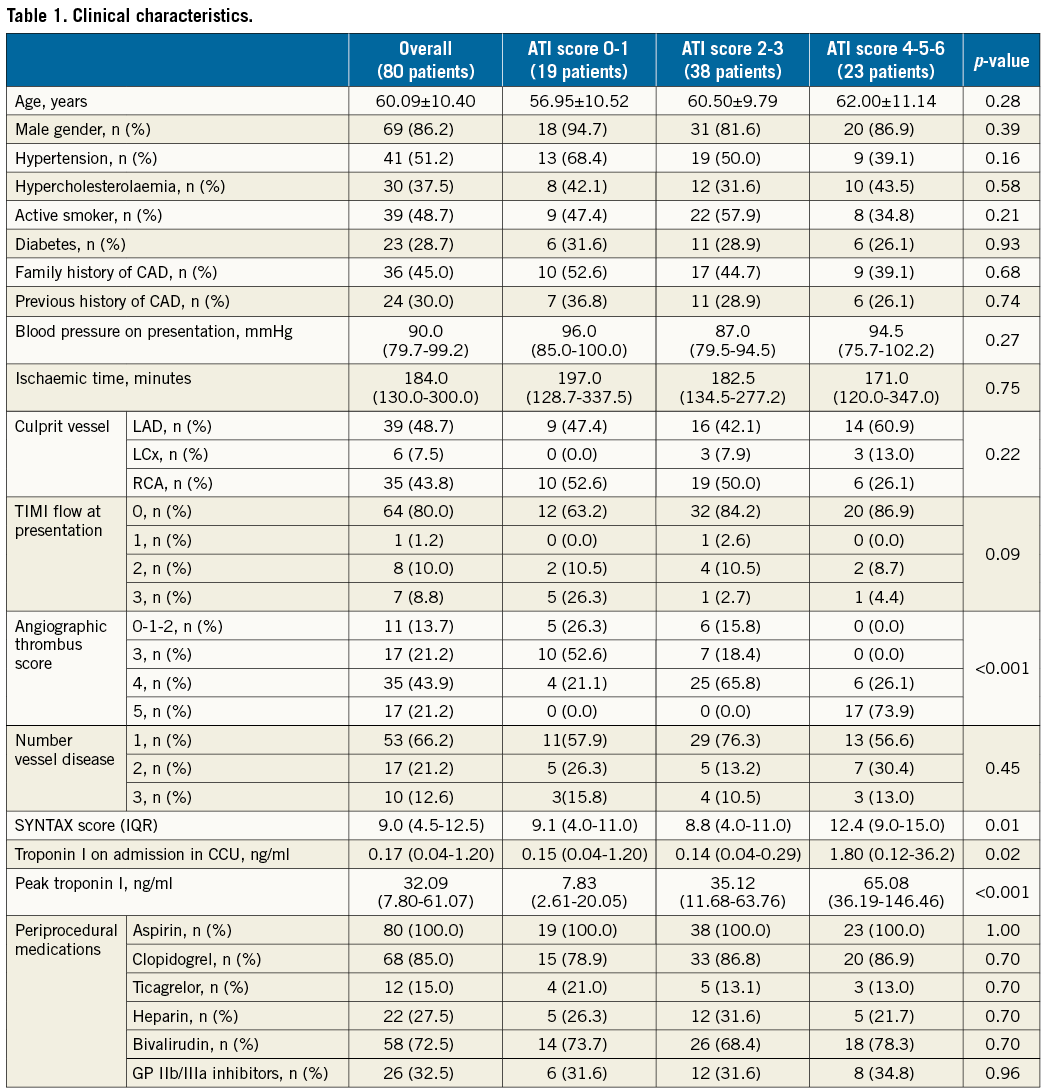
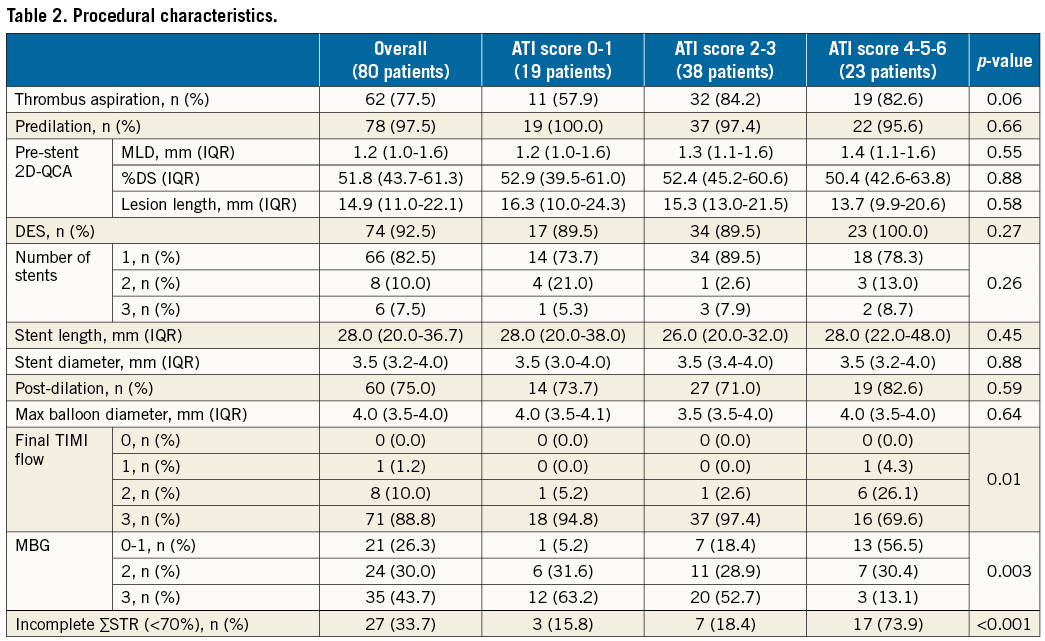
Notably, patients with a high ATI score had a lower rate of post-pPCI TIMI 3 flow (p=0.01), a lower rate of MBG 3 (p=0.003) and a higher rate of incomplete ∑STR (p<0.001) (Table 2).
ATI SCORE AND INDEX OF MICROVASCULAR RESISTANCE
Interestingly, the ATI score maintained its accuracy in detecting a post-procedural IMR >40 (Figure 2A) with an area under the curve (AUC) of 0.88 (95% CI: 0.80-0.96, p<0.001). This result was maintained after exclusion of the 20 patients previously included in the retrospective ATI score validation (Figure 2B).

Figure 2. ATI score and post-pPCI IMR. A) The scatter plot reports the distribution of the post-pPCI values of IMR according to ATI score. The dotted red line corresponds to the post-procedural IMR cut-off of 40. B) The ROC analysis with the corresponding AUC is reported for ATI score in detecting a post-pPCI IMR >40 for the overall cohort and after the exclusion of the initial 20 patients previously included in the retrospective validation of the ATI score.
ATI SCORE AND POST-pPCI CMRI
Patients with higher ATI score were more likely to have larger IS% (ATI0-1: 16.5% [8.7-22.9] vs. ATI2-3: 28.5% [15.5-35.0] vs. ATI4-5-6: 35.5% [22.2-44.4], p=0.001, p for linear trend <0.001) (Figure 3A) and greater degree of MVO% (ATI0-1: 0.0% [0.0-0.9] vs. ATI2-3: 0.7% [0.0-2.5] vs. ATI4-5-6: 4.1% [1.2-10.7], p<0.001, p for linear trend <0.001) (Figure 3B). Patients in the higher risk subgroup also had a higher rate of intramyocardial haemorrhage at cMRI (ATI0-1: 21.0% vs. ATI2-3: 31.6% vs. ATI4-5-6: 78.3%, p<0.001) (Figure 3C) and a lower degree of myocardial salvage index (ATI0-1: 50.0% [30.8-66.4] vs. ATI2-3: 40.9% [24.2-51.1] vs. ATI4-5-6: 24.4% [17.3-37.0], p=0.003, p for linear trend=0.001) (Figure 3D).
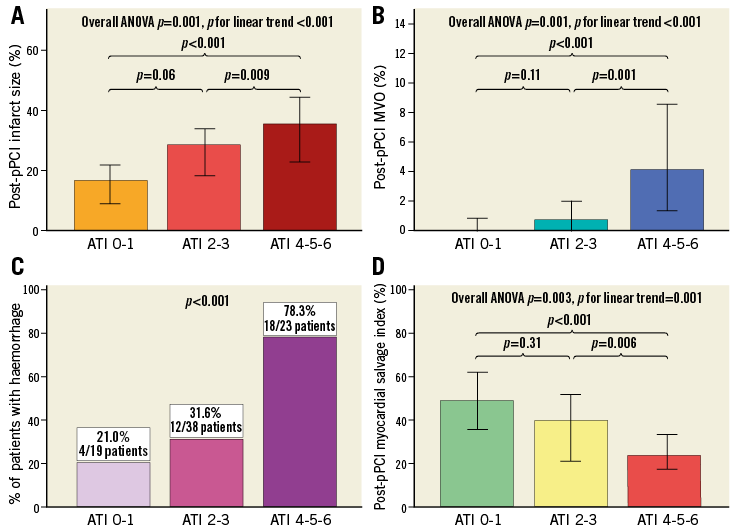
Figure 3. ATI score and cMRI post pPCI. A) Correlation between ATI score risk groups and the extent of IS. B) Correlation between ATI score risk groups and the extent of MVO. C) Distribution of the rate of intramyocardial haemorrhage detected at cMRI in the three ATI score risk categories. D) Correlation between ATI score risk groups and the degree of myocardial salvage. In panels A, B and D, data are represented as median values with 95% confidence intervals. In panel C, bars correspond to frequencies expressed as percentage. IS: infarct size; MVO: microvascular obstruction
ATI SCORE AND SIX-MONTH cMRI
In the 50 patients imaged at six-month follow-up with cMRI, a similar correlation between ATI score and IS% was observed, with a larger IS% in patients with high ATI score (ATI0-1: 12.7% [4.1-18.0] vs. ATI2-3: 16.3% [8.0-24.7] vs. ATI4-5-6: 29.5% [19.9-49.5], p=0.02, p for linear trend=0.01) (Figure 4A). Interestingly, a significant reduction in IS% between baseline and six-month follow-up was observed in patients in the ATI0-1 and ATI2-3 groups (p=0.01 for ATI0-1 and p=0.001 for ATI2-3), but not for patients in the ATI4-5-6 group (p=0.47) (Figure 4B). The degree of IS shrinkage was significantly greater for patients with low ATI score (ATI0-1: 31.8% [18.9-64.1] vs. ATI2-3: 30.2% [17.1-50.2] vs. ATI4-5-6: 13.6% [2.1-23.1], p=0.03, p for linear trend=0.01) (Figure 4C). In the end, a significant trend towards negative left ventricular remodelling was observed in patients with a high ATI score, the end-diastolic volume percentage variation being 2.7% (–4.5-12.1) in ATI0-1, 3.5% (–1.8-11.5) in ATI2-3 and –9.2% (–20.2-4.4) in ATI4-5-6 (p=0.008, p for linear trend=0.02) (Figure 4D).

Figure 4. ATI score and six-month cMRI. A) Correlation between ATI score risk groups and the extent of IS. B) Variation in IS between baseline and six-month follow-up in the three ATI score risk categories. C) Correlation between ATI score risk groups and degree of IS shrinkage. D) Correlation between ATI score risk groups and EDV variation with a clearer trend towards negative remodelling in the high ATI score group. The dotted line represents the 20% cut-off value of EDV percentage change to diagnose adverse negative remodelling. In all panels data are represented as median values with 95% confidence intervals. EDV: end-diastolic volume; IS: infarct size
PREDICTORS OF INFARCT SIZE
At multivariable analysis, only LAD as culprit vessel (β=0.34, p=0.001) and ATI score (β=0.42, p=0.03) were independently associated with post-procedural IS% (R2=0.40). When the analysis was repeated in the 50 patients who underwent six-month cMRI follow-up, only LAD as culprit vessel (β=0.28, p=0.03) and ATI score (β=0.45, p=0.001) were significantly related to six-month IS% (R2=0.33).
Discussion
Early identification of STEMI patients at risk of poor post-pPCI myocardial reperfusion is desirable, as high-risk patients identified at a very early stage, before stenting and before completion of the procedure, may be considered for additional therapies or alternative strategies. In the current study, we demonstrate the ability of the ATI score to predict final IS and extent of MVO measured by cMRI.
We have previously proposed the ATI score as a tool that can be used in the catheterisation laboratory, prior to stenting, to identify STEMI patients at the highest risk of microvascular injury3. The score is simply derived and incorporates age, angiographic TS and pre-stenting IMR. It has been derived from data collected from an initial cohort of STEMI patients and appears to predict accurately a post-procedural IMR greater than 40. In the current study, we confirm these results by again observing the ability of the ATI score to predict a post-procedural IMR greater than 40 in this further independent cohort, reporting the percentage risk of final IMR >40 in the three ATI score categories (ATI0-1; ATI2-3; ATI4-5-6) and with a ROC analysis showing an AUC of 0.88.
When considering the derivation of the ATI score, one might consider that it was inherently biased in predicting final IMR, given that an integral component of the scoring system is (pre-stenting) IMR itself. Notably, final post-stenting IMR has previously been shown to predict IS, as assessed by cMRI15, and to be related to clinical prognosis4. Consequently, the current study aimed to expand the application of the score to independent outcome measures assessed by cMRI. We observed that the three ATI score risk categories were able to predict angiographic and electrocardiographic indices of myocardial reperfusion, observing a lower rate of TIMI 3, MBG 3 and complete ∑STR in patients in the highest ATI score group. These data were then expanded by comparing the ATI risk score categories with the gold standard technique for IS and MVO detection represented by cMRI.
In this study, we demonstrated that the ATI score correlates with IS and MVO extent in the acute phase after pPCI with a significant linear trend across the three ATI score risk groups. Additionally, the ATI score predicts the presence of intramyocardial haemorrhage and thus the degree of severity of microvascular injury, which was more frequent in the ATI4-5-6 risk group. The observation of a lower myocardial salvage index in patients with high ATI score further confirms the notion that patients in this group gain less benefit from revascularisation using a conventional approach with stenting. This observation emphasises and mitigates the need for the application of additional or alternative approaches.
These results observed in the acute phase are extended using longer-term imaging follow-up. In the subgroup of patients with a six-month cMRI follow-up, the ATI score correlated with six-month IS extension in patients in the ATI4-5-6 group. This may have potential clinical and prognostic implications, particularly as those patients in the high ATI risk group had a lower degree of IS shrinkage and a trend towards EDV dilation and negative remodelling at six-month follow-up, despite there being no differences across the three ATI score risk groups in terms of procedural, periprocedural treatment and post-discharge medical therapy. Consequently, the ATI score appears to predict acute and long-term IS extent independently and may have a role in tailoring not only the revascularisation strategy in the catheterisation laboratory but potentially also the medical therapy delivered in the short and medium term.
The value of the ATI score resides essentially in three main factors. Firstly, it is the first stratification tool for STEMI patients including a parameter of coronary physiology, thus assessing directly the coronary microvasculature at a very early stage. IMR can theoretically offer a more accurate assessment of coronary microvasculature when compared to other variables such as MBG or ∑STR. Moreover, because IMR is measured after flow restoration, this does not imply any delay in revascularisation or prolongation of door-to-balloon time. Secondly, since it is based on the combination of thrombotic burden and microvasculature status assessment, the ATI score presents an appealing pathophysiological explanation for slow/no reflow, as the underlying microvascular injury is known to be the result of the interaction(s) of distal embolisation, individual susceptibility, ischaemia injury and reperfusion injury16. The ATI score could take into consideration all these aspects since thrombotic burden may account for the likelihood of distal embolisation, while IMR may address individual susceptibility, ischaemia and reperfusion injury17.
Ultimately, the ability to predict adverse outcomes accurately in the early phase of STEMI treatment is only of real value if additional therapies can be applied to improve clinical outcomes. We believe that scoring systems, such as the ATI score, which can be applied in the very earliest stage of the revascularisation procedure once blood flow has been restored and prior to stenting, are vital, given that subsequent stent implantation is associated with most distal embolisation18. Such risk scores will help to determine the role, prior to stenting, of alternative strategies that may include deferred stenting, administration of established or novel antiplatelet agents, vasodilators or application of new devices.
Limitations
A possible limitation of the ATI score is that pre-stenting IMR was not corrected for coronary wedge pressure19. Although possible, measuring wedge pressure adds an additional procedural step and we have previously shown that non-corrected IMR agrees closely with IMR corrected for coronary wedge pressure6. Another limitation is the non-core lab-based quantification of angiographic TS. However, TS within the ATI score has the highest weight when equal to five. This is readily identified since it is based on the presence of flow after the guidewire passage and we doubt that this would be reclassified by a core lab assessment. A further limitation of the study is represented by a 37% drop-off at six-month follow-up, with only 50 patients having six-month cMRI.
A final element potentially perceived as a further limitation is the requirement for IMR measurement before stenting. Premising that its safety and feasibility has been largely described in STEMI3,10, it is interesting to highlight that the introduction of new-generation pressure guidewires with improved manoeuvrability and “steerability” represents a major development, facilitating the application of the ATI score as an early stratification tool in STEMI.
Conclusions
The current study demonstrates the role of the ATI score as a stratification tool applicable early in the revascularisation procedure for STEMI. Although further evidence needs to be collected in terms of clinical outcome data, the score is a promising tool for clinical application in future STEMI trials of novel therapies.
| Impact on daily practice The ATI score has been shown to detect high-risk STEMI patients early in the catheterisation laboratory and before stenting, and helps to predict short- and medium-term clinical outcome. The ATI score may play a role in guiding the adoption of both current and novel additional or alternative therapeutic strategies both within the catheterisation laboratory and in the per- and post-procedural phases. It might have a further application in the field of clinical research, as a possible inclusion criterion for future clinical trials investigating devices and therapies for STEMI. |
Funding
This study was supported by the British Heart Foundation (BHF; grant CH/16/1/32013), the BHF Centre of Research Excellence, Oxford (RG/13/1/30181), and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre.
Conflict of interest statement
A. Banning has received an unrestricted research grant from Boston Scientific. The other authors have no conflicts of interest to declare.

