Abstract
Myocardial tissue perfusion remains compromised in 30-40% of patients with ST-segment elevation myocardial infarction (STEMI) despite restored epicardial patency after primary percutaneous coronary intervention (pPCI). This phenomenon is attributed to microvascular dysfunction secondary to numerous pathophysiological mechanisms, including distal embolisation of plaque and thrombus material. Its association with larger post-infarction myocardial necrosis, impaired left ventricular recovery, and worse clinical outcome illustrates the pertinence of a comprehensive armamentarium for the diagnosis, protection and treatment of microvascular dysfunction in STEMI patients. Current strategies to protect the microvasculature during pPCI are based on the assumption that distal embolisation of thrombotic and atheromatous debris is the main mechanism precipitating impaired myocardial tissue perfusion. However, recent findings suggest that this assumption is only true for the border zone of the ischaemic myocardium, whereas the infarct core consists of intramyocardial haemorrhage secondary to microvascular destruction, rather than obstruction. This observation has pertinent implications for contemporary and future adjuvant treatment strategies in STEMI patients. In this review, we provide an overview of the currently available armamentarium to assess the microvasculature, review contemporary strategies in pPCI to protect the myocardium, and discuss novel insights into microvascular pathophysiology that may help guide our focus from the coronary arteries to the microvasculature.
Introduction
ST-segment elevation myocardial infarction (STEMI) management has evolved dramatically, now encompassing dedicated STEMI networks, potent antithrombotic drugs, rapid achievement of reperfusion, and advanced secondary prevention programmes, which has resulted in a decline in morbidity and mortality in STEMI patients. However, it is well recognised that myocardial tissue perfusion remains compromised in 30-40% of STEMI patients, despite rapid and successful mechanical revascularisation. This phenomenon is associated with larger post-infarction myocardial necrosis, which is a major determinant of morbidity and mortality in STEMI survivors.
Impaired microvascular reperfusion is considered the consequence of numerous pathophysiological mechanisms, including reperfusion injury, distal embolisation of plaque and thrombus material, endothelial dysfunction, leucocyte plugging, and external compression of the microvasculature. Its clinical presentation may range from sudden absence of coronary flow, “no-reflow”, to mild flow impairment only appreciated with advanced diagnostic modalities. The clinical pertinence of this phenomenon has triggered tremendous efforts in translational and clinical research in search of a comprehensive armamentarium for the diagnosis, protection and treatment of coronary microvascular dysfunction in the setting of STEMI. In this review, we provide an overview of the currently available armamentarium to assess the microvasculature, review contemporary strategies in pPCI to protect the myocardium, and discuss novel insights into microvascular pathophysiology that may help guide our focus from the coronary arteries to the microvasculature.
Coronary microvasculature: assessing the “black box”
The coronary arterial vasculature comprises the epicardial coronary arteries and the myocardial microvasculature, consisting of extramyocardial prearterioles and intramyocardial arterioles. In normal physiological conditions, the epicardial conductance vessels offer little resistance and predominantly fulfil a capacitance function. The extramyocardial prearterioles, with diameters ranging from 100 to 500 µm, alleviate alterations in perfusion pressure by flow-dependent dilatation and thereby maintain coronary flow within narrow ranges at the origin of the arterioles. The concomitant alterations in the vascular tone of the intramyocardial arterioles, with diameters less than 100 µm, are predominantly regulated by the release of metabolites by the myocardium in response to an increase in oxygen consumption.
At present, no techniques are available that allow in vivo evaluation of the coronary microcirculation in humans, which has consequently long been considered the “black box” of the coronary circulation. While semi-quantitative assessment of the microvasculature by means of angiography-derived parameters has governed assessment of epicardial and microvascular perfusion after pPCI, more advanced measures of microvascular function have emerged over the last decade – both invasive tools for direct assessment of microvascular function in the catheterisation laboratory, as well as non-invasive tools to assess functional microvascular abnormalities subacutely.
Invasive methods
CORONARY ANGIOGRAPHY
Angiography-derived parameters are commonly used to assess reperfusion success after primary PCI, with the Thrombolysis In Myocardial Infarction (TIMI) flow grade as the most widely adopted angiographical surrogate for epicardial flow restoration. However, even optimal TIMI flow may be associated with impaired myocardial tissue perfusion as identified by the myocardial blush grade (MBG) or intracoronary flow velocity measurements, indicating the limited sensitivity of epicardial contrast flow as a surrogate for microvascular function and integrity1-3. Despite improved sensitivity of MBG for impaired tissue perfusion, direct invasive assessment of coronary flow using sensor-tipped guidewires remains the most sensitive approach to assess impaired coronary flow3.
INVASIVE PHYSIOLOGY TECHNIQUES: CORONARY FLOW
Intracoronary blood flow velocity (CFV) measurements using the Doppler flow technique have been available since the early 1990s, providing Doppler-derived coronary flow velocity reserve (CFVR) as well as morphological characteristics of the flow velocity envelope, diastolic deceleration time (DDT) and early systolic retrograde flow (SRF) (Figure 1). These parameters, assessed in the infarct-related coronary artery, have consistently been shown to correspond to the extent of microvascular dysfunction after reperfusion for STEMI and to be associated with the extent of post-infarction myocardial necrosis and microvascular obstruction (MVO)4, as well as with recovery of ventricular function and subsequent long-term clinical outcome5-7. Moreover, microvascular dysfunction also occurs in perfusion territories remote from the infarcted tissue6, the extent of which is associated with impaired long-term clinical outcome8.
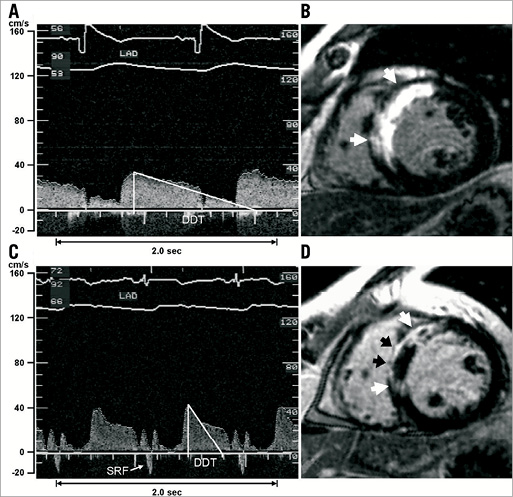
Figure 1. Coronary flow velocity recordings and corresponding late gadolinium-enhanced LGE images of patients without and with microvascular injury (MVI). The coronary flow velocity spectrum (A) of Patient #1 shows antegrade systolic flow without early systolic retrograde flow (SRF) and a normal diastolic deceleration time (DDT). A corresponding LGE image (B) shows transmurally infarcted myocardium (white arrows) in the anteroseptal wall without signs of MVI. The flow velocity pattern (C) of Patient #2 demonstrates SRF and short DDT. The LGE image (D) shows transmurally infarcted myocardium (white arrows) with substantial MVI (black arrows). LAD: left anterior descending coronary artery. (Reproduced with permission4).
Alternatively, the thermodilution technique was applied to assess coronary flow invasively, in which the temperature sensitivity of a pressure-sensor-equipped guidewire is exploited to measure the mean transit time of a bolus of cold saline injected down a coronary artery (Figure 2)9. Whereas this technique only allowed assessment of coronary flow reserve, lacking the potential to evaluate the morphological characteristics of the flow velocity envelope, a recent study has suggested that the shape of the thermodilution curve corresponds to distal microvascular functional status10.
Figure 2. Thermodilution-derived coronary flow using temperature-sensitive pressure-sensor-equipped guidewires (St. Jude Medical, St. Paul, MN, USA) to assess the coronary flow reserve (CFR) and index of microcirculatory resistance (IMR).
INVASIVE PHYSIOLOGY TECHNIQUES: MICROVASCULAR RESISTANCE PARAMETERS
Measuring both distal coronary pressure and a surrogate of coronary flow, either Doppler flow velocity or thermodilution-derived mean transit time, allows the selective evaluation of microvascular resistance to coronary blood flow11,12. The minimal microvascular resistance, assessed by the hyperaemic microvascular resistance index (HMR) for Doppler-derived coronary flow (Figure 3) or index of microcirculatory resistance (IMR) for thermodilution-derived coronary flow (Figure 2), is notably associated with ventricular recovery and clinical outcome after STEMI6,13. The additional assessment of microvascular resistance during basal, autoregulated conditions allows study of the pathophysiological behaviour of the coronary microcirculation in the setting of STEMI6,8. These approaches have shown that, in the acute setting of reperfused STEMI, minimal microvascular resistance is transitorily increased throughout the heart, due to the effects of ischaemia, reperfusion, and neurohumoral activation. After recovery of microvascular vasodilatory function, disturbed microvascular function is typically recognised by the magnitude of microvascular resistance in basal conditions, indicating a persistent stress on the autoregulated mechanism that is associated with impaired clinical outcome6,8. In general, the major advantage of microvascular assessment directly in the catheterisation laboratory is to enable immediate instigation of adjunctive therapeutic strategies.
Figure 3. Combined pressure and Doppler flow velocity recordings to assess the coronary flow velocity reserve (CFVR) and the hyperaemic microvascular resistance index (HMR) using the ComboMap® system (Volcano Corp., San Diego, CA, USA).
Non-invasive methods
The non-invasive assessment of microvascular function notably provides important prognostic information, and has allowed critical insights into pathophysiological mechanisms and consequences of impaired microcirculatory perfusion. Non-invasive assessment of the coronary microvasculature may be performed using positron emission tomography (PET), cardiovascular magnetic resonance (CMR), or even echocardiography14. In general, non-invasive techniques allow quantification of myocardial blood flow with the aid of radiolabelled or paramagnetic tracers, measuring the tracer enhancement in the myocardium.
PET provides a non-invasive absolute quantification of regional myocardial tissue perfusion if appropriate tracers and mathematical models are applied15. Moreover, sophisticated kinetic modelling and advances in the imaging armamentarium eliminated the necessity of establishing a normal reference region of interest in patients with an increased heterogeneity in myocardial perfusion, as present in the setting of STEMI6,16. In addition to absolute perfusion, coronary vascular resistance can be estimated by combining non-invasively derived myocardial blood flow with (mean) arterial blood pressure. Although this method intrinsically does not allow differentiation between flow impairment originating from microvascular dysfunction and (residual) epicardial obstruction17, hybrid imaging systems combining PET and CT offer concomitant evaluation of functional and anatomical integrity of the coronary circulation, providing opportunities for advanced hydrodynamic modelling to identify the origin of myocardial flow impairment.
The high anatomical detail of contrast-enhanced CMR has allowed the identification of zones within the infarcted myocardium, with areas of hyperenhancement reflecting infarct size and a dark hypoenhanced core considered to reflect microvascular obstruction (MVO). These characteristics related to MVO have been consistently associated with impairment of invasive parameters of microvascular function, impaired recovery of ventricular function, and adverse clinical outcome. Hence, the occurrence of MVO on CMR has been used as a surrogate efficacy endpoint for novel therapeutic strategies that target protection of microvascular function in STEMI14.
From microvascular obstruction to microvascular injury: a paradigm shift in treatment strategies?
Current strategies to protect the microvasculature during pPCI for STEMI are based on the assumption that distal embolisation of epicardial thrombotic and atheromatous debris is the main mechanism precipitating MVO and no-reflow. This assumption has led to the general inference that the contrast-devoid core of gadolinium-enhanced CMR images represents MVO, and the use of its magnitude as a surrogate of therapeutic efficacy. However, a comprehensive translational study correlating CMR and histological data in a porcine STEMI model with CMR data obtained in STEMI patients undergoing pPCI recently suggested other concomitant pathophysiological mechanisms. The assumption of obstruction as the cause of the lack of contrast uptake was found to be true for the border zone of the infarcted myocardium, but the contrast-devoid core of the infarcted tissue was shown to represent intramyocardial haemorrhage secondary to microvascular destruction, rather than obstruction (Figure 4)18,19. In detail, the authors identified a border zone of the infarct core with morphologically intact microvasculature that contained microthrombi, and an infarct core with extensive necrosis, loss of vascular integrity and erythrocyte extravasation. These characteristics can be attributed to the ischaemic transmural wavefront, which originates in the subendocardium and progressively moves towards the subepicardial myocardium20. At the border of the ischaemic wavefront, injured vessels can still receive and produce coagulation factors, explaining why microthrombi were found, and adding a cause of MVO besides embolisation21,22. The loss of cellular integrity in the infarct core is due to complex, interconnected mechanisms following pronounced ischaemia caused by the acute loss of epicardial patency23. These hypoxia-induced mechanisms disrupt the endothelial barrier and compromise microvasculature integrity, facilitating extravasation of blood cells upon reperfusion24,25. This interpretation of CMR characteristics, previously related to MVO, as a combination of an obstructed border zone and a damaged core led the authors to refer to this condition as microvascular injury (MVI).
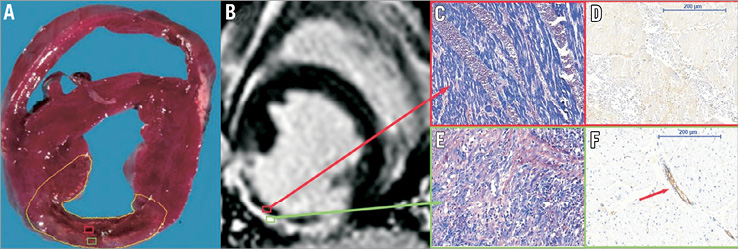
Figure 4. Histology of the porcine model. A) An infarct core (red frame) surrounded by an infarct border zone (green frame). The core corresponds to late gadolinium-enhanced (LGE) images (B) with the area known as “microvascular obstruction”. Microscopy reveals extensive haemorrhage on phosphotungstic acid-haematoxylin staining (C, magnification ×200) with a complete loss of the vascular integrity on anti-CD31 staining (D). The border zone corresponds with the enhanced area on LGE (B): this area contains myocyte necrosis, leucocyte influx, and granulation tissue on phosphotungstic acid-haematoxylin (E), with intact vessels on anti-CD31 staining (F, magnification ×200), of which some are plugged by microthrombi (arrow). (Reproduced with permission19).
Contemporary epicardial revascularisation strategies: a focus on protection of the microcirculation
Strategies to protect the microcirculation adopted in contemporary STEMI care are focused on embolic and thrombotic impairment of tissue perfusion to reduce infarct size and improve outcomes, and include the use of thrombus aspiration, novel anticoagulants, adjuvant anticoagulation regimens, and advanced stent designs.
THROMBUS ASPIRATION
In order to avoid distal thrombus embolisation, potentially associated with MVO and the no-reflow phenomenon, thrombus aspiration has emerged as a simple, rapid, and relatively inexpensive adjunct to pPCI26-30. Thrombus aspiration was shown to improve TIMI flow grade, MBG, as well as clinical outcome31-33. However, recent large-scale randomised trials have cast doubts on the clinical benefit of routine thrombus aspiration. To date, TAPAS is the only large randomised controlled trial suggesting that routine thrombus aspiration before stenting during pPCI results in improved clinical outcome31. INFUSE-AMI was the first to indicate that routine thrombus aspiration was not associated with a reduction in 30-day infarct size, suggesting no protective effect on the microvascular level, even in large anterior wall STEMI patients34. Moreover, the 7,244-patient registry-based randomised TASTE trial showed that routine thrombus aspiration before pPCI did not reduce 30-day mortality as compared to pPCI alone35. Long-term results of TASTE are eagerly awaited, and the ongoing TOTAL trial (NCT01149044) will provide additional insights into the benefit of routine thrombus aspiration.
ADVANCED STENT DESIGNS
Despite excellent performance of contemporary stent platforms, several new-generation coronary stents with a focus on STEMI treatment are currently being evaluated, in which stent designs are shifting towards prevention of distal embolisation in stenting of thrombus-rich epicardial lesions. An illustrative example is a bare metal stent covered with a micronet mesh (MGuard™; InspireMD, Tel Aviv, Israel) , designed to mitigate distal embolisation and associated no-reflow by trapping embolism-prone material within the mesh. In pPCI for STEMI, its use resulted in superior rates of restored epicardial coronary flow and complete ST-segment resolution compared with conventional stents36. A large randomised clinical trial evaluating its effects on infarct size is ongoing (NCT01869738). Self-expanding coronary stents are also being proposed to limit distal embolisation since they do not require aggressive balloon dilatation to acquire optimal expansion. Nonetheless, in clinical practice, balloon dilatation is regularly performed either pre- or post-stent placement to prevent acute complications associated with severe malappositioning37. It seems conceivable that balloon dilatation may reduce some of the benefit related to self-expansion, but this remains speculative.
In the recent DEFER-STEMI trial, stent placement was deferred if TIMI 3 flow was obtained after thrombus aspiration with or without low-pressure balloon angioplasty38. This approach was governed by the hypothesis that immediate stent placement is associated with adverse effects such as MVO and no-reflow, whereas delayed stenting allows partial relief of thrombus burden and recovery of microvascular function. Indeed, delayed stenting reduced the rate of no-reflow, and improved myocardial salvage as compared to an immediate stenting strategy, paving the way for this novel approach, but also for dedicated stent platforms.
PROCEDURAL AND ADJUNCTIVE ANTICOAGULATION REGIMENS
Aggressive anticoagulation regimens governed pharmacological adjunctive strategies in the acute setting of pPCI, due to the consideration of distal embolisation of thrombus fragments as the main determinant of MVO and no-reflow.
The use of fibrinolysis as a standalone therapeutic approach in STEMI has declined dramatically after extensive evidence showed inferiority to pPCI. However, the administration of low-dose intracoronary streptokinase immediately after pPCI was shown to improve TIMI flow grade and microvascular function, to limit infarct size, and to preserve left ventricular volumes and function39,40. These hypothesis-generating findings emphasise the potential of adjunctive fibrinolytic therapy to improve microvascular function, and justify further exploration in more definitive trials41,42.
The potent inhibition of platelet aggregation with the concomitant use of glycoprotein IIb/IIIa inhibitors and heparin was found to reduce the incidence of ischaemic events over the use of heparin alone, albeit with concerns of haemorrhagic complications43-45. Consequently, the administration of a glycoprotein IIb/IIIa inhibitor should be considered for bail-out therapy if there is angiographic evidence of massive thrombus, slow or no-reflow, or a thrombotic complication according to the European guidelines33, and the US guidelines consider it reasonable to administer glycoprotein IIb/IIIa inhibitors in patients with STEMI undergoing PCI, although they do not definitively recommend it as routine therapy32.
The direct thrombin inhibitor bivalirudin was suggested to provide potent anticoagulation similar to the combination of heparin and a glycoprotein IIb/IIIa inhibitor, but resulting in reduced rates of haemorrhagic complications, overall improving both early and long-term clinical outcomes46,47. Notwithstanding, recent trials have cast doubts on the efficacy of bivalirudin to improve outcome in contemporary practice using novel thienopyridines in combination with heparin or bivalirudin alone, and glycoprotein IIb/IIIa inhibition in a bail-out strategy. Interestingly, the INFUSE-AMI trial showed that, in patients with large anterior wall STEMI undergoing pPCI with bivalirudin anticoagulation, administration of a glycoprotein IIb/IIIa inhibitor directly to the infarct lesion site prior to pPCI was associated with a significant reduction in 30-day infarct size34.
Future strategies to protect the microvasculature
Amid the focus on preventing embolic and thrombotic complications of pPCI to protect the microvasculature, the observation of MVI has pertinent implications for adjuvant treatment strategies in STEMI, and could well explain the limited efficacy of contemporary treatment strategies focused on improving microvascular perfusion. The identification of a border zone of the infarct core comprising morphologically intact microvasculature containing microthrombi suggests an obstructive or at least thrombotic origin that might benefit from thrombus aspiration and aggressive anticoagulation therapy secondary to revascularisation. However, an infarct core without intact vasculature characterised by excessive extravasation suggests a haemorrhagic origin that might be aggravated by aggressive anticoagulation therapies. Additionally, the vulnerability of the hypoxic microvasculature could augment the loss of vessel integrity in response to the sudden restoration of perfusion pressure by pPCI, which corroborates the fact that reperfusion itself has been shown to induce expansion of myocardial necrosis. Moreover, inflammatory processes that go beyond the embolisation theory have been described as leading to infarct expansion48.
Many novel therapeutic strategies to protect the microvasculature have been evaluated in experimental and clinical models, most of them unfortunately without much success to date. In their review, Windecker et al concluded that reduction of treatment delays remains the cornerstone of preventing myocardial injury in view of the limited novel therapeutic options to reduce microvascular injury49. In addition, the cardioprotective effectiveness of interruption of reperfusion with short periods of ischaemia, also referred to as postconditioning, remains a subject of debate. The new insights into MVI together with previously documented benefits of gradual reperfusion indicate that the destructive force of abrupt restoration of perfusion pressure and the vulnerability of the ischaemic microvasculature could well be therapeutic targets for future treatment strategies50-52. The growing insights into the detrimental effects of the inflammatory response after STEMI provide another potential therapeutic target, even more so since it has been shown that early intravenous beta-blockade therapy reduces infarct size and increases left ventricular ejection fraction, likely due to alteration of the inflammatory response following ischaemia48,53.
Conclusion
Myocardial reperfusion goes beyond restoring epicardial patency. Advanced diagnostic modalities allow accurate assessment of microvascular function and the effects of reperfusion in the setting of STEMI, which allows the identification of patients who may benefit from adjuvant therapies after pPCI. Future therapeutic strategies should not limit their therapeutic target to resolving distal embolisation of the microvasculature, but should focus on protecting the microvasculature against the harmful effects of reperfusion and on enhancing healing of the injured microvasculature and myocardium in those patients who may benefit most.
Conflict of interest statement
The authors have no conflicts of interest to declare.

