Abstract
The major challenge in the treatment of ST-elevation myocardial infarction (STEMI) is not only restoration of normal coronary blood flow but also microvascular perfusion. In fact, both electrocardiographic (ST segment resolution) and angiographic measures of myocardial perfusion (myocardial blush grade) have been shown to predict mortality after primary percutaneous coronary intervention (PPCI). Initial enthusiasm for manual thrombectomy arose after the apparent mortality benefit observed in the TAPAS trial (N=1,071). Meta-analyses of small trials suggest that manual thrombectomy improves epicardial and microvascular perfusion with trends towards benefit for survival. On the other hand, meta-analyses of small trials of mechanical thrombectomy show improvement in ST resolution without an effect on survival. Recently, the TASTE trial (N=7,244) showed no reduction in mortality with manual thrombectomy but trends toward reduction in rates of rehospitalisation for recurrent MI and stent thrombosis. The interpretation of TASTE should be cautious given that the trial had a much lower than expected mortality, and modest but important treatment effects cannot be excluded (20-30%). The largest trial, the ongoing TOTAL trial (N=10,700), will help determine the effect of manual thrombectomy for important clinical outcomes. A planned individual patient meta-analysis of the TOTAL and TASTE trials will have approximately 17,000 patients to examine the effect on clinical outcomes.
Scientific rationale for thrombectomy
ST-elevation myocardial infarction is characterised by plaque rupture and occlusion of the infarct artery with thrombus. Modern primary PCI is very effective in restoring epicardial flow in the infarct artery but a significant limitation is distal embolisation of thrombus and obstruction of the microvasculature1-3.
Microvascular perfusion in STEMI, as measured by angiographic myocardial blush grade (MBG) and electrocardiographic ST-segment resolution, has clearly been linked to mortality after primary PCI (Figure 1)3. About one third of patients who have undergone PPCI have impaired microvascular perfusion despite restoration of normal epicardial flow, and they have a significantly increased mortality. Furthermore, thrombus burden during primary PCI has been linked to mortality4. In an analysis of 812 consecutive STEMI patients, large as compared to small thrombus burden patients had higher mortality (12.9% vs. 7.8% at one year, p=0.025), higher rates of stent thrombosis (8.2% vs. 1.3%, p=0.001) and lower rates of grade 3 MBG (35% vs. 53%, p<0.001)4.
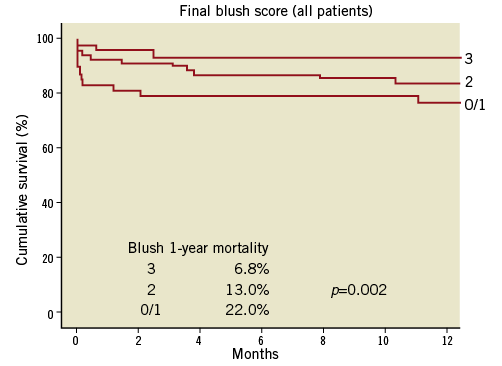
Figure 1. Myocardial blush grade and mortality after PPCI. (Reproduced with permission3).
Thrombectomy has the potential to reduce distal embolisation and improve microvascular perfusion during PPCI. In addition, thrombectomy in STEMI allows better visualisation of the infarct artery and lesion by removing thrombus, which is useful for treating the culprit lesion and determining stent length. Residual thrombus at the target lesion may be a nidus for stent thrombosis, and therefore thrombus removal may lower the risk of stent thrombosis. Furthermore, removal of thrombus may prevent late stent malapposition and subsequent stent thrombosis. There is an intuitive biologic rationale for thrombectomy based on these potential benefits.
Types of thrombectomy
The two main types of thrombectomy are manual aspiration and mechanical rheolytic thrombectomy. In clinical practice, thrombectomy is most commonly performed using simple manual aspiration devices with a tapered microcatheter attached to a syringe. Mechanical thrombectomy (e.g., using the AngioJet® Ultra thrombectomy system; Bayer Healthcare/MEDRAD, Inc., Warrendale, PA, USA) is characterised by active mechanical suction and high-velocity saline jets to break up thrombus. Manual thrombectomy catheters are significantly less expensive and simpler to use than mechanical thrombectomy devices.
Common predictors for failure of successful thrombectomy with manual thrombectomy devices include tortuosity of the coronary artery, a calcified lesion, and a bifurcation lesion5. Failure to cross or reach the lesion has been observed in about 10% of the patients5. No aspirate has been observed in about 10-20%2,5,6. Finally, manual thrombectomy devices may not be able to remove very large thrombi. Other potential complications of thrombus aspiration include dissection of the coronary artery and thrombus dislodgement during retrieval of the aspiration catheter which may embolise thrombus to the non-infarct vessel. Finally, operator technique can impact on the success of thrombectomy and Table 1 shows the optimal technique for thrombus aspiration7.

The newest generation of aspiration catheters has achieved improved deliverability by using a stylet-based system (e.g., Export Advance™ aspiration catheter; Medtronic, Minneapolis, MN, USA; Eliminate™ aspiration catheter; Terumo Corp., Tokyo, Japan). These devices may improve the ability to cross tortuosity and retrieve thrombus.
Evidence for manual thrombectomy
Meta-analyses of randomised trials have demonstrated that manual thrombectomy improves epicardial and microvascular perfusion and reduces distal embolisation in STEMI8,9. The first large trial of manual thrombectomy was the TAPAS (N=1,071, single-centre trial) which randomised patients to the Export aspiration catheter (Medtronic, Minneapolis, MN, USA), vs. PCI alone in STEMI2. They showed an improvement in primary outcome of MBG 0 or 1 (17% vs. 26%, p<0.001), but this modest change in a surrogate outcome led to a near 50% reduction in cardiac mortality at one year (3.6% vs. 6.7%, p=0.020)2,10.
The TAPAS trial led to the design of the TASTE trial (N=7,244), a registry-based randomised trial in Sweden comparing manual thrombectomy vs. PCI alone in patients with STEMI11. The primary outcome of mortality was not different (2.8% vs. 3.0%, p=0.63), but there were trends towards reduction in re-hospitalisation for MI (0.5% vs. 0.9%, p=0.09) and stent thrombosis (0.2% vs. 0.5%, p=0.06). Importantly, despite being the largest trial ever performed in thrombectomy, the trial had fewer deaths than planned (213 vs. planned 456), so the trial cannot exclude realistic but important reductions in mortality (20-30%). Finally, the one year results of TASTE are not yet available and will provide important long-term data on thrombectomy.
Future ongoing trials
The TOTAL trial is a randomised trial of nearly 11,000 patients which is designed to determine if routine manual thrombectomy vs. PCI alone can reduce the composite of cardiovascular death, MI, shock or class IV heart failure at 180 days12. The trial is an event-driven trial and is powered to detect a 20% relative risk reduction in primary outcome. There are important substudies of the TOTAL trial, including the optical coherence tomography (OCT) substudy that will compare thrombus burden prior to stenting in thrombectomy group vs. PCI alone and provide insights into the effectiveness of current technology to remove thrombus. Finally, there is a planned individual patient meta-analysis of both the TASTE and TOTAL trials and this will have significantly more power for the outcome of mortality with approximately 17,000 patients.
Another question is, does selective thrombectomy in patients with a high thrombus burden compared to no thrombectomy improve clinical outcomes? The subgroup analysis of TASTE found no mortality benefit in patients with high thrombus burden (n=2,216)11; however, further subgroup analysis from a larger trial, TOTAL, and individual patient meta-analyses will have much more power to examine this question.
Evidence for mechanical thrombectomy
One significant limitation of manual aspiration is its inability to remove large volume thrombus. Mechanical thrombectomy, if more effective at thrombus removal, may be postulated to be particularly beneficial in patients with large thrombus. A randomised trial of manual vs. mechanical thrombectomy demonstrated less residual thrombus with mechanical thrombectomy as measured by OCT13. The two largest trials of mechanical thrombectomy vs. PCI alone show divergent results. The AIMI trial (N=480) demonstrated, in an all-comer STEMI population, that the AngioJet (Bayer Healthcare/MEDRAD, Inc.) was associated with increased infarct size and a higher rate of mortality compared to PCI alone14. On the other hand, in selected patients with high thrombus burden, the JETSTENT trial (N=501) demonstrated improved ST resolution and MACE but no difference in mortality15. In patients with high thrombus burden, a large-scale trial is needed with mechanical thrombectomy to determine its benefit on clinical outcomes.
Based on the current evidence, mechanical thrombectomy remains a bail-out option in cases of very high thrombus burden not responsive to manual thrombectomy or other measures.
Guidelines
The ESC provides a class IIa recommendation for routine manual thrombectomy during STEMI16. On the other hand, there are no ESC guideline recommendations regarding mechanical thrombectomy.
Summary and recommendations for clinical practice
Manual thrombectomy remains a useful tool during STEMI PCI but further trials will help determine its effect on important clinical events. In the meantime, manual thrombectomy should be considered simply another tool in the PCI toolkit to optimise an angiographic result during PPCI. Selective use of these devices in patients with large thrombus burden to facilitate stent placement appears to be a logical approach until further data are available. Finally, regarding mechanical thrombectomy, future trials are needed to determine the benefit/risk ratio in patients with high thrombus burden.
Conflict of interest statement
S. Jolly reports grant support from Medtronic, and honoraria from AstraZeneca and St. Jude Medical. K Niemela has no conflicts of interest to declare.

