Abstract
Background: The optimal strategy to prevent no-reflow in ST-elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention (PCI) is unknown.
Aims: We aimed to examine the effect of thrombectomy on the outcome of no-reflow in key subgroups and the adverse clinical outcomes associated with no-reflow.
Methods: We performed a post hoc analysis of the TOTAL Trial, a randomised trial of 10,732 patients comparing thrombectomy versus PCI alone. This analysis utilised the angiographic data of 1,800 randomly selected patients.
Results: No-reflow was diagnosed in 196 of 1,800 eligible patients (10.9%). No-reflow occurred in 95/891 (10.7%) patients randomised to thrombectomy compared with 101/909 (11.1%) in the PCI-alone arm (odds ratio [OR] 0.95, 95% confidence interval [CI]: 0.71-1.28; p-value=0.76). In the subgroup of patients who underwent direct stenting, those randomised to thrombectomy compared with PCI alone experienced less no-reflow (19/371 [5.1%] vs 21/216 [9.7%], OR 0.50, 95% CI: 0.26-0.96). In patients who did not undergo direct stenting, there was no difference between the groups (64/504 [12.7%] vs 75/686 [10.9%)], OR 1.18, 95% CI: 0.82-1.69; interaction p-value=0.02). No-reflow patients had a significantly increased risk of experiencing the primary composite outcome (cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or NYHA Class IV heart failure) at 1 year (adjusted hazard ratio 1.70, 95% CI: 1.13-2.56; p-value=0.01).
Conclusions: In patients with STEMI treated by PCI, thrombectomy did not reduce no-reflow in all patients but may be synergistic with direct stenting. No-reflow is associated with increased adverse clinical outcomes.
Introduction
Coronary no-reflow is defined as myocardial hypoperfusion resulting from microvascular obstruction in the presence of a patent epicardial circulation1. In the primary percutaneous coronary intervention (PCI) era for ST-elevation myocardial infarction (STEMI), the no-reflow phenomenon is increasingly recognised as a poor prognostic marker for left ventricular remodelling, infarct size, left ventricular ejection fraction, and long-term mortality2. Despite its clinical importance, no preventive or treatment strategies for no-reflow have demonstrated significant benefits on hard clinical outcomes234.
The Trial of Routine Aspiration Thrombectomy with PCI Versus PCI Alone in Patients With STEMI Undergoing Primary PCI (TOTAL) was a randomised controlled trial comparing routine upfront manual aspiration thrombectomy versus primary PCI alone5. The trial showed no difference for the primary composite outcome of cardiovascular death, myocardial infarction (MI), cardiogenic shock, or New York Heart Association (NYHA) Class IV heart failure between the groups; although, there was an increased risk of stroke in the thrombectomy group67.
We investigated whether being randomised to thrombectomy versus PCI alone affected the angiographic core laboratory-adjudicated outcome of no-reflow in key subgroups. Furthermore, we explored the association between no-reflow and major cardiovascular outcomes at 1 year and determined significant clinical predictors of no-reflow.
Methods
Population and study design
The TOTAL Trial was an international, multicentre randomised trial in which 10,732 patients (87 centres, 20 countries) with STEMI were randomly (1:1) assigned to undergo routine upfront manual aspiration thrombectomy (using the Export catheter; Medtronic) versus PCI alone. The trial protocol and results have been previously published58. The angiographic parameters of a randomly selected subset of patients were assessed at the angiographic core laboratory at the Peter Munk Cardiac Care Centre, Toronto, Canada, where the investigators were blinded to all non-angiographic patient characteristics8. The study complies with the Declaration of Helsinki and was approved by the ethics committee at each participating centre. All patients provided written informed consent before enrolment.
Definitions and endpoints
No-reflow was defined as a post-PCI (measured at the end of the procedure) reduction in coronary flow, characterised by a Thrombolysis in Myocardial Infarction (TIMI) flow <3 in the absence of dissection, thrombus, spasm, or high-grade residual stenosis at the original target lesion39. No-reflow was determined by the operators during the initial procedure and subsequently by the blinded core laboratory personnel for the angiographic core laboratory subset.
The primary composite outcome for the present analysis included cardiovascular mortality, recurrent myocardial infarction, cardiogenic shock, or NYHA Class IV heart failure, as per the TOTAL Trial5. Other outcomes included the individual components of the composite endpoint: all-cause mortality, all-cause rehospitalisation, and stent thrombosis. The definitions of these endpoints have been previously described in detail58. A central committee blinded to study group allocation adjudicated all outcome events.
Angiographic core laboratory analysis
Quantitative coronary angiographic analysis was performed using QAngio XA version 7.3 (Medis Medical Imaging). As previously described, the epicardial coronary flow was reported using the qualitative TIMI classification, while thrombus grades were defined according to Gibson1011.
Statistical analysis
We used the modified intention-to-treat data from the TOTAL Trial to include only patients who had undergone randomisation and primary PCI. The primary study population was the angiographic core laboratory subset. For comparison, analyses were also performed for the entire TOTAL population, in which the operator assessed no-reflow.
We presented continuous data with normal distribution as means with standard deviations and used the Student’s t-test for statistical testing. Non-normal data were presented as medians with interquartile ranges and compared using the Wilcoxon rank-sum test. We assessed dichotomous variables using chi-square analysis and Fisher’s exact test.
Logistic regression with randomisation to thrombectomy as the exposure and no-reflow status as the outcome across multiple subgroups with interaction terms was used to calculate odds ratios (OR) and 95% confidence intervals (CI). The key subgroups were chosen from the initial TOTAL Trial and subject matter knowledge. The key subgroups included TIMI thrombus grade (<4 vs ≥4), symptom onset (<6 hours vs 6-12 hours), initial TIMI flow (0-1 vs 2-3), primary PCI operator volume (mean and below vs above mean), MI type (anterior vs non-anterior), age (≤65 years vs >65 years), and direct stenting (yes vs no)7.
We calculated unadjusted and adjusted hazard ratios (HR) and 95% CI using an inverse probability of treatment-weighted (IPTW) Cox proportional hazards model for the clinical outcomes defined above. The adjusted model included age, sex, previous MI, Killip class ≥2, anterior MI, presenting systolic blood pressure, initial TIMI thrombus grade, and initial TIMI flow. No violation of the proportional hazard modelling choice was detected using Schoenfeld residuals. We plotted the standardised differences before and after IPTW modelling. We also plotted the unadjusted and adjusted marginal survival functions according to no-reflow status for the primary composite outcome. We used multiple imputations (5 datasets) using fully conditional specification methods for missing data12. In the case of missing data, missing values for each covariate were <1%.
Multivariable predictors of no-reflow were identified using a stepwise selection algorithm with a significance level of <0.10 for entry and exit in a logistic regression model. We included age, sex, hypertension, diabetes mellitus, smoking status, previous MI, Killip class, anterior MI, presenting heart rate, presenting systolic and diastolic blood pressure, initial TIMI thrombus grade (<4 vs ≥4), initial TIMI flow (0-1 vs 2-3), prophylactic use of GPIIb/IIIa inhibitors and vasodilators, operator experience by primary PCI volume, actual thrombectomy use, and direct stenting.
To investigate the interrater reliability between angiographic core laboratory analysis and operator assessment, we constructed a 2x2 table and calculated Cohen’s κ coefficient. A 2-sided p-value of <0.05 was considered significant for all analyses. We did not adjust for multiple comparisons. Results were obtained using R software, version 4.1.3 (R Foundation for Statistical Computing).
Results
Angiographic core laboratory analysis
A total of 1,800 patients from the angiographic core laboratory analysis were included, with 196 (10.9%) patients experiencing the no-reflow phenomenon (Figure 1). The patients in the no-reflow group were older (64.4 years vs 60.8 years); however, both groups had a similar proportion of female patients (25.0% [no-reflow] vs 22.1% [without no-reflow]) (Table 1). Patients with no-reflow had a higher Killip class, lower pre-PCI TIMI flow, and less direct stenting.
No-reflow occurred in 95/891 (10.7%) patients randomised to thrombectomy compared with 101/909 (11.1%) in the PCI-alone arm (OR 0.95, 95% CI: 0.71-1.28; p=0.76) (Figure 2). In the subgroup of patients who underwent direct stenting, those randomised to thrombectomy compared with PCI alone experienced less no-reflow (19/371 [5.1%] vs 21/216 [9.7%], OR 0.50, 95% CI: 0.26-0.96). In patients who did not undergo direct stenting, there was no difference between groups (64/504 [12.7%] vs 75/686 [10.9%], OR 1.18, 95% CI: 0.82-1.69; interaction p-value=0.02). The effect of thrombectomy on no-reflow remained consistent across all other subgroups. Of note, the effect of thrombectomy on no-reflow did not change according to the infarct-related artery (Supplementary Figure 1).
At 1 year, STEMI patients with no-reflow had a 70% increased relative risk of experiencing the primary composite outcome of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or NYHA Class IV heart failure (31/196 [15.8%] vs 101/1,604 [6.3%], adjusted HR 1.70, 95% CI: 1.13-2.56; p-value=0.01) compared with patients without no-reflow (Table 2, Figure 3). Furthermore, no-reflow was also associated with an adjusted increased risk of cardiogenic shock (16/196 [8.2%] vs 28/1,604 [1.7%], HR 2.20, 95% CI: 1.14-4.24; p-value=0.02). The IPTW modelling provided adequate covariate balance with all absolute standardised mean differences reduced to less than 0.1 (Supplementary Figure 2).
As demonstrated in Figure 4, the 3 significant predictors of no-reflow were age (per 10-year increment) (OR 1.25, 95% CI: 1.09-1.42; p-value=0.001), pre-PCI TIMI flow 3 (OR 0.33, 95% CI: 0.19-0.56; p-value<0.0001), and direct stenting (OR 0.66, 95% CI: 0.45-0.95; p-value=0.03).
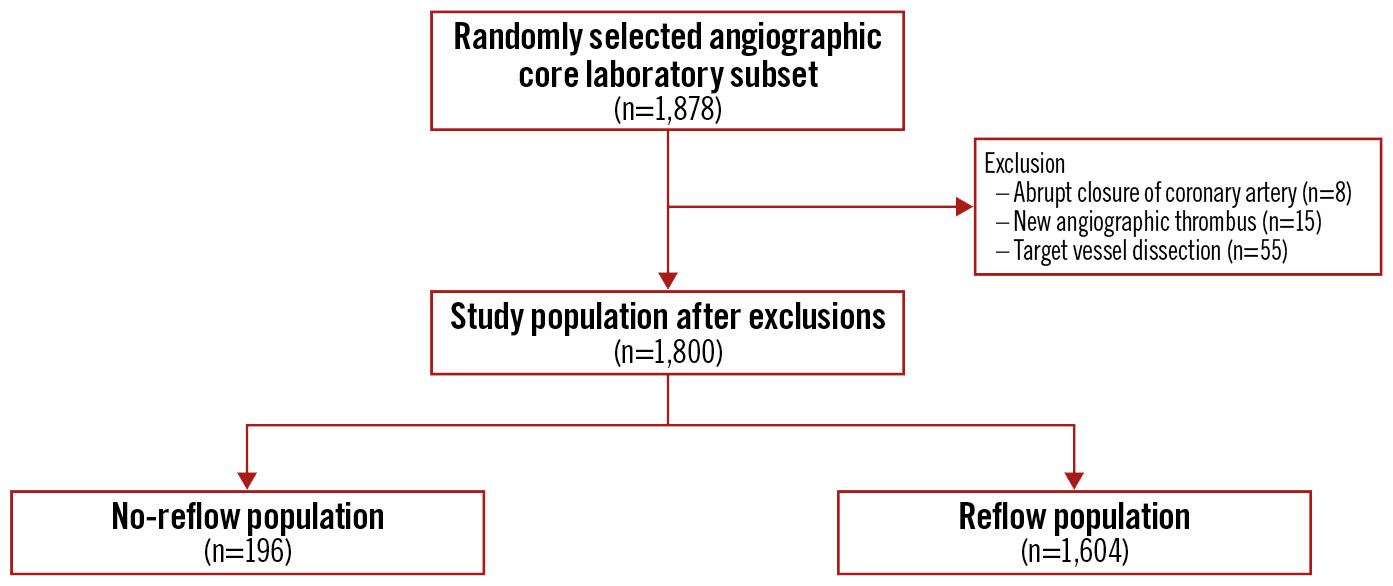
Figure 1. CONSORT diagram for the angiographic core laboratory population according to no-reflow statistics.
Table 1. Baseline and procedural characteristics for the angiographic core laboratory patients.
| Variable | No-reflow (n=196) | Reflow (n=1,604) | p-value | |
|---|---|---|---|---|
| Demographic and comorbidities | ||||
| Age, years | 64.4±12.9 | 60.8±11.8 | <0.01 | |
| Female sex | 49 (25.0) | 354 (22.1) | 0.40 | |
| Hypertension | 104 (53.1) | 823 (51.3) | 0.69 | |
| Diabetes mellitus | 40 (20.4) | 287 (17.9) | 0.44 | |
| Current smoker | 63 (32.1) | 752 (46.9) | <0.01 | |
| Previous MI | 20 (10.2) | 172 (10.7) | 0.92 | |
| Previous PCI | 14 (7.1) | 148 (9.2) | 0.41 | |
| Clinical presentation | ||||
| Killip class ≥2 | 16 (8.1) | 68 (4.2) | 0.02 | |
| Location of myocardial infarction | Anterior | 93 (47.4) | 626 (39.0) | 0.05 |
| Inferior | 98 (50.0) | 908 (56.6) | ||
| Lateral or other | 5 (2.6) | 70 (4.4) | ||
| Presenting heart rate, beats per minute* | 79.2±19.4 | 76.8±17.7 | 0.10 | |
| Presenting systolic blood pressure, mmHg* | 135.0±25.1 | 134.1±24.3 | 0.63 | |
| Presenting diastolic blood pressure, mmHg* | 81.7±16.1 | 82.5±16.1 | 0.86 | |
| Periprocedural medication use | ||||
| Unfractionated heparin | 160 (81.6) | 1,196 (74.6) | 0.04 | |
| Bivalirudin | 32 (16.3) | 260 (16.2) | 1.0 | |
| Low molecular weight heparin | 55 (28.1) | 406 (25.3) | 0.46 | |
| Intracoronary vasodilator | Upfront | 19 (9.7) | 131 (8.2) | 0.33 |
| Bailout | 22 (11.2) | 53 (3.3) | <0.01 | |
| Glycoprotein IIb/IIIa inhibitor | Upfront | 48 (24.5) | 474 (29.6) | 0.14 |
| Bailout | 46 (23.5) | 247 (15.4) | <0.01 | |
| Intracoronary bolus | 34 (36.2)1 | 280 (38.8)1 | 0.97 | |
| Initial thrombus grade | ||||
| 0: no thrombus present | 8 (4.1) | 73 (4.6) | <0.01 | |
| 1: possible thrombus present | 4 (2.0) | 135 (8.4) | ||
| 2: definite thrombus present, <0.5x vessel diameter | 9 (4.6) | 77 (4.8) | ||
| 3: definite thrombus present, 0.5-2.0x vessel diameter | 5 (2.6) | 120 (7.5) | ||
| 4: definite thrombus present, >2.0x vessel diameter | 28 (14.3) | 248 (15.5) | ||
| 5: total occlusion | 138 (70.4) | 946 (59.0) | ||
| Missing data | 4 (2.0) | 5 (0.03) | ||
| TIMI flow before PCI | ||||
| TIMI flow 0 | 142 (72.4) | 960 (59.9) | <0.01 | |
| TIMI flow 1 | 24 (12.2) | 119 (7.4) | ||
| TIMI flow 2 | 18 (9.2) | 191 (11.9) | ||
| TIMI flow 3 | 10 (5.1) | 330 (20.6) | ||
| Missing data | 2 (1.0) | 4 (0.2) | ||
| Thrombectomy | 91 (46.4) | 820 (51.1) | 0.98 | |
| Presence of macroscopic aspirate material retrieved with thrombectomy catheter | 60 (65.9) | 539 (65.7) | 0.91 | |
| Use of stenting | ||||
| Stenting | 169 (86.2) | 1,552 (96.8) | <0.01 | |
| Direct stenting2 | 40 (23.7) | 547 (35.2) | <0.01 | |
| Stenting with DES2 | 91 (53.8) | 822 (53.0) | 0.95 | |
| PCI procedure time, mins | 43 [30.0-60.5] | 37 [27.0-50.0] | <0.01 | |
| Data are mean±SD, n (%) or median [IQR]. 1The denominator is based on the number of patients who received a glycoprotein IIb/IIIa inhibitor. 2The denominator is based on the number of patients who received a stent. *Less than 1% missing data. DES: drug-eluting stent; IQR: interquartile range; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; TIMI: Thrombolysis in Myocardial Infarction | ||||
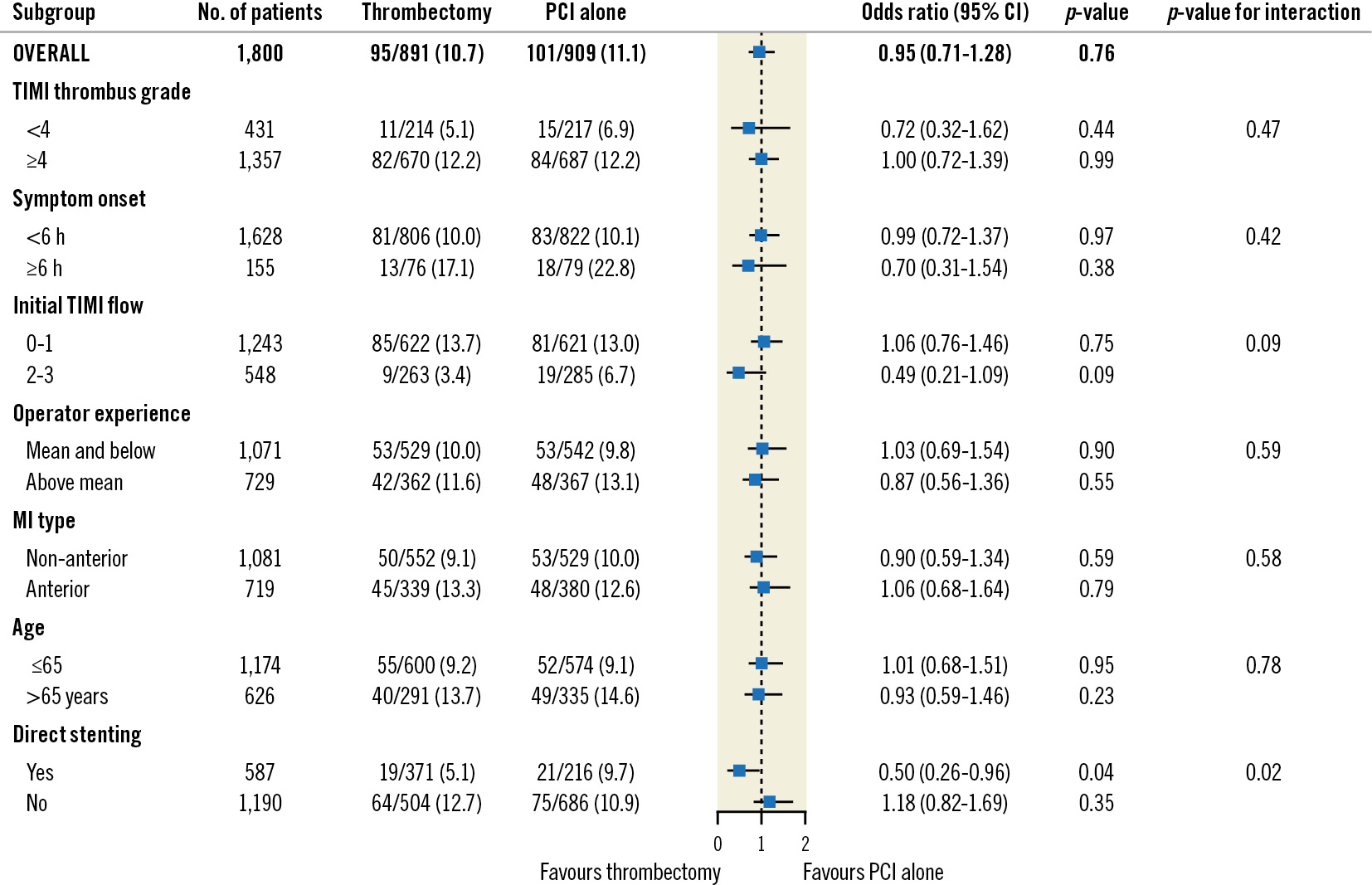
Figure 2. Randomised subgroup analysis for no-reflow in the angiographic core laboratory population. CI: confidence intervals; MI: myocardial infarction; PCI: percutaneous coronary intervention; TIMI: Thrombolysis in Myocardial Infarction
Table 2. Major adverse outcomes according to no-reflow status at 1 year for the angiographic core laboratory patients.
| Variables | No-reflow (n=196) | Reflow (n=1,604) | Unadjusted HR (95% CI) | Adjusted HR1 (95% CI) | p-value |
|---|---|---|---|---|---|
| Primary composite outcome | |||||
| Cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or NYHA Class IV heart failure | 31 (15.8) | 101 (6.3) | 2.69 (1.80-4.02) | 1.70 (1.13-2.56) | 0.01 |
| Other outcomes | |||||
| All-cause mortality | 17 (8.7) | 44 (2.7) | 3.31 (1.89-5.79) | 1.54 (0.90-2.66) | 0.12 |
| Cardiovascular mortality | 14 (7.1) | 34 (2.1) | 3.51 (1.88-6.55) | 1.67 (0.88-3.19) | 0.12 |
| Recurrent myocardial infarction | 7 (3.6) | 43 (2.7) | 1.34 (0.60-2.98) | 1.16 (0.28-4.74) | 0.81 |
| NYHA Class IV heart failure | 8 (4.1) | 27 (1.7) | 2.52 (1.14-5.56) | 1.63 (0.69-3.86) | 0.25 |
| Cardiogenic shock | 16 (8.2) | 28 (1.7) | 4.89 (2.65-9.04) | 2.20 (1.14-4.24) | 0.02 |
| All-cause rehospitalisation | 8 (4.1) | 40 (2.3) | 1.71 (0.80-3.64) | 1.25 (0.63-2.51) | 0.52 |
| Stent thrombosis | 6 (3.1) | 27 (1.7) | 1.93 (0.80-4.67) | 1.36 (0.35-5.23) | 0.62 |
| Data are n (%). 1C-statistic=0.57 (95% CI: 0.53-0.61). CI: confidence interval; HR: hazard ratio; NYHA: New York Heart Association | |||||
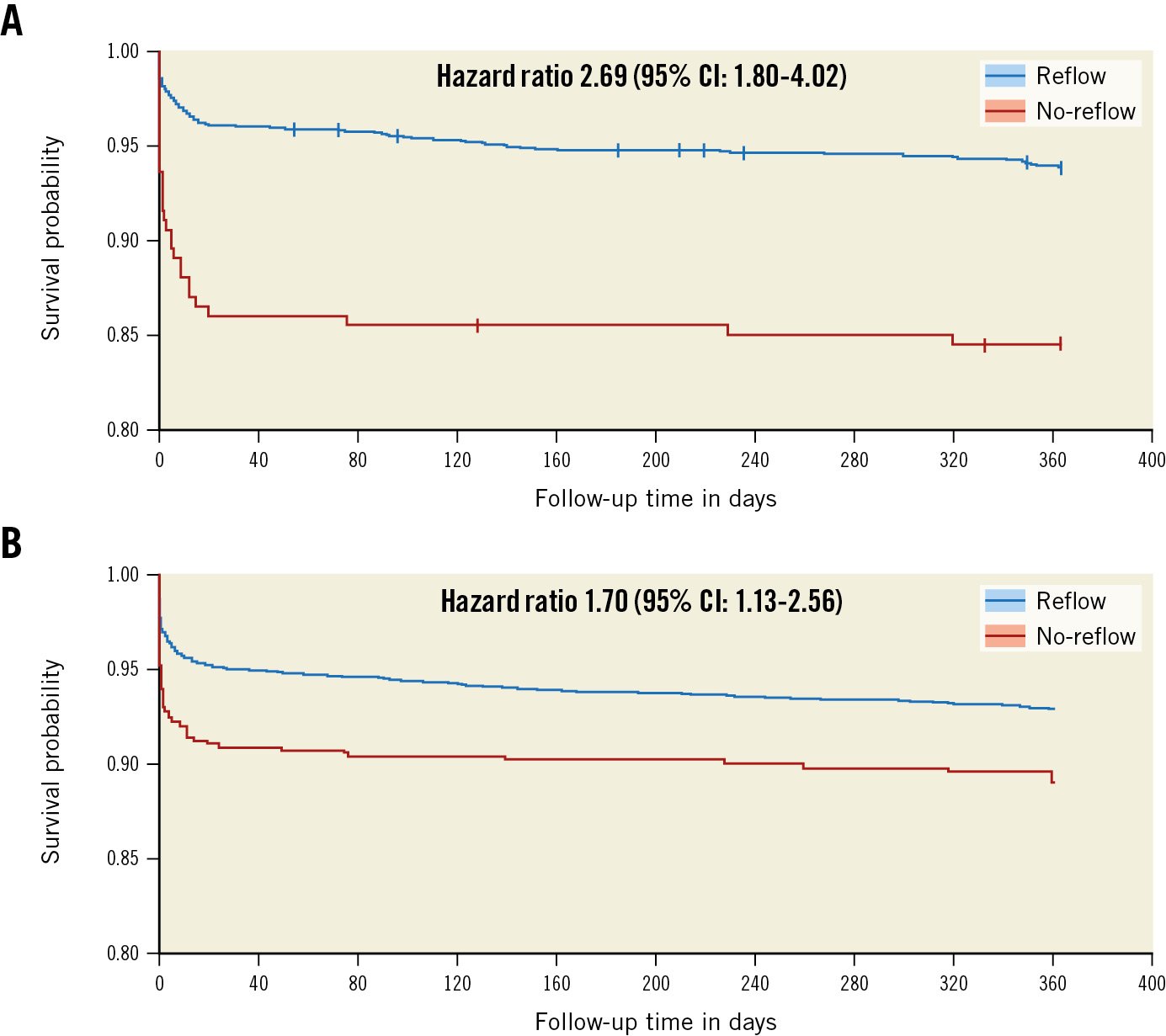
Figure 3. Survivor probability for the composite primary outcome according to reflow status in the angiographic core laboratory population. A) Unadjusted survivor function and B) adjusted marginal survivor function. CI: confidence interval
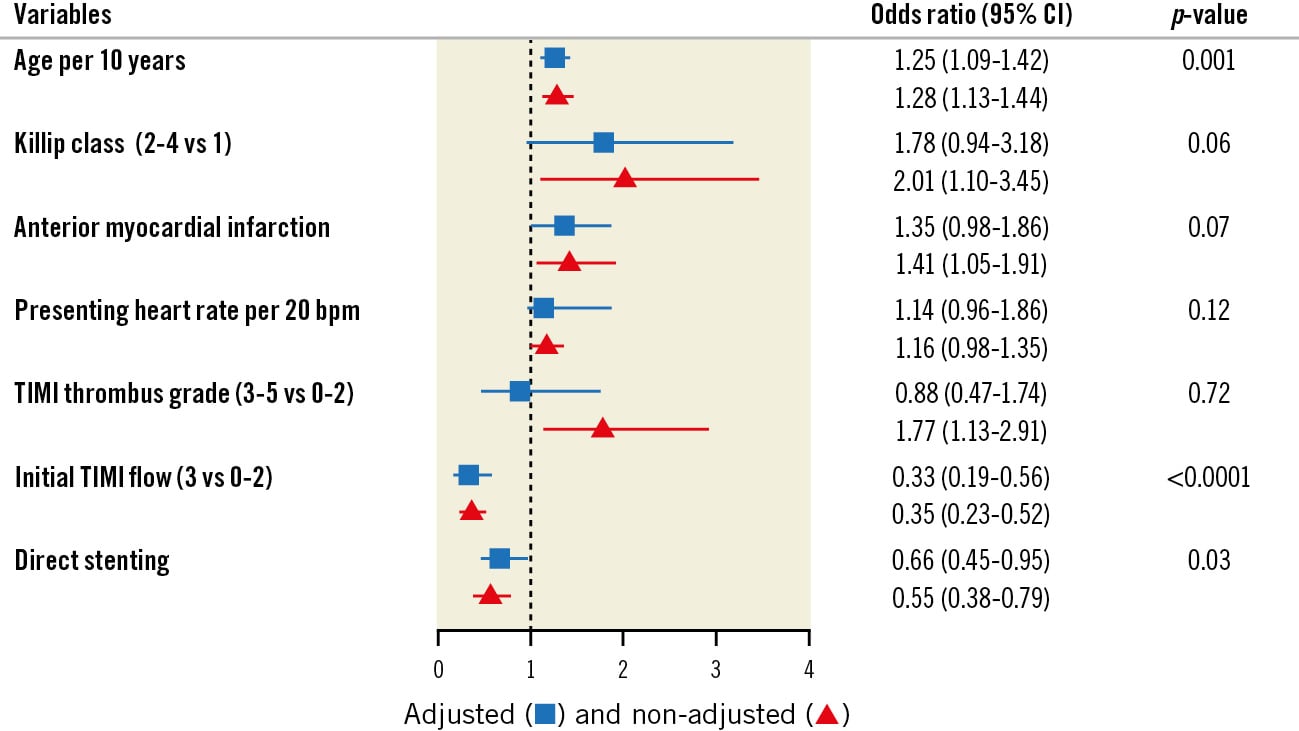
Figure 4. Independent predictors of no-reflow in the angiographic core laboratory population. C-statistic=0.67 (95% CI: 0.63-0.71) for the multivariable logistic regression model. CI: confidence interval; TIMI: Thrombolysis in Myocardial Infarction
Investigator-reported no-reflow
The baseline characteristics of the operator-assessed patients were similar to those of the angiographic core laboratory patients, as demonstrated in Supplementary Table 1. According to operator assessment, 644 of the 9,755 (6.6%) eligible patients were deemed to have no-reflow compared with 10.9% in the angiographic core laboratory review (Supplementary Table 2, Supplementary Figure 3). Cohen’s κ value of 0.29 demonstrates only fair interrater agreement (Table 3).
In the operator-assessed no-reflow population, the randomised subgroup analysis demonstrated that the effect of thrombectomy remained consistent across all subgroups with no significant interaction with direct stenting (Supplementary Figure 4). However, direct stenting remained a strong predictor of no-reflow (OR 0.66, 95% CI: 0.53-0.69; p-value<0.0001) (Supplementary Figure 5).
After IPTW adjustment (Supplementary Figure 6), the operator-assessed no-reflow patients had a significantly increased risk of experiencing the primary composite outcome (104/644 [16.1%] vs 679/9,111 [7.5%], HR 1.73, 95% CI: 1.33-2.25; p-value≤0.01), all-cause mortality (79/644 [12.3%] vs 335/9,111 [3.7%], HR 2.39, 95% CI: 1.73-3.30; p-value≤0.01), cardiovascular mortality (72/644 [11.2%] vs 274/9,111 [3.0%], HR 2.60, 95% CI: 1.85-3.66, p-value≤0.01) and cardiogenic shock (44/644 [6.8%] vs 174/9,111 [1.9%], HR 2.41, 95% CI: 1.68-3.46; p-value≤0.0001) at 1 year compared to patients without no-reflow (Supplementary Table 2).
Table 3. Agreement between operator assessment and angiographic core laboratory assessment for post-PCI no-reflow.
| Operator assessment | ||||
|---|---|---|---|---|
| Angiographic core lab assessment | Reflow | No-reflow | Total | |
| Reflow | 1,486 (85.7) | 74 (4.3) | 1,560 (89.9) | |
| No-reflow | 122 (7.0) | 53 (3.0) | 175 (10.1) | |
| Total | 1,608 (92.7) | 127 (7.3) | 1,735 | |
| Data are n (%). McNemar’s chi-squared test p<0.01; Cohen’s κ =0.29. PCI: percutaneous coronary intervention | ||||
Discussion
Data from the angiographic core laboratory of the largest randomised trial of manual thrombus aspiration during primary PCI in STEMI demonstrated that routine use of manual thrombus aspiration did not reduce the incidence of no-reflow. However, no-reflow was reduced in the subgroup of patients who received direct stenting following thrombus aspiration. Finally, these analyses confirm that no-reflow is common, prognostically important, and associated with adverse events in the current era of primary PCI. We demonstrated that no-reflow is associated with adverse outcomes, which is confirmatory; our randomised analysis showed that thrombectomy is not associated with a reduction in no-reflow and that direct stenting may have a synergistic effect with thrombectomy in an angiographic core laboratory-adjudicated population, which is novel.
There have been mixed results regarding the use of direct stenting to prevent no-reflow in STEMI. A meta-analysis of randomised trials of direct stenting in myocardial infarction (n=754) demonstrated that direct stenting did not significantly improve no-reflow (6.6% in the direct-stenting group compared to 6.9% in the conventional-stenting group, OR 0.78, 95% CI: 0.39-1.55; p=0.48)13. However, trials that combined thrombectomy with direct stenting versus standard PCI alone were excluded. The Polish-Italian-Hungarian RAndomized ThrombEctomy Trial (PIHRATE) demonstrated that a thrombectomy and direct-stenting strategy decreased no-reflow after primary PCI compared to a predilatation strategy without thrombectomy in a STEMI population14. While similar, our comparison is slightly different; our randomised data suggest that thrombectomy may be associated with a decreased risk of no-reflow in patients undergoing direct stenting, compared to direct stenting alone. Thrombus aspiration likely facilitates direct stenting. Although prior studies did not find an interaction between thrombus aspiration and direct stenting, they used investigator-reported no-reflow rather than reports from angiographic core laboratories15. Similarly, we caution against overinterpreting the absence of significant interaction between direct stenting and thrombectomy in the operator-assessed population, as we have demonstrated that operator-assessed no-reflow may be unreliable and may lead to patient misclassification (κ=0.29). A similar interrater agreement for no-reflow has been previously reported16. Our study may be used as a cautionary tale, underlining the importance for studies analysing angiographic markers of no-reflow to use blinded core angiographic laboratory-adjudicated data as much as possible to avoid biased findings.
Our finding that no-reflow is associated with a markedly increased relative risk for adverse clinical outcomes at 1 year is comparable to previous studies1718. In an observational study by Ndrepepa et al, STEMI patients who experienced no-reflow had a larger infarct size (15% vs 8.0% of the left ventricle; p<0.001) and had an increased risk of 5-year mortality (adjusted HR 1.66, 95% CI: 1.17-2.36; p=0.004)18. When blood cannot circulate through the necrotic myocardium, macrophages cannot clear cellular debris, and humoral factors cannot access the tissue, thus, disrupting the optimal myocardial healing processes19. Consequently, no-reflow leads to maladaptive remodelling, left ventricular dysfunction, and a higher incidence of adverse events.
More than 10% of our study population developed no-reflow. Given the poor prognosis associated with no-reflow, clinicians may benefit from early recognition of the risk factors for no-reflow. Age and initial TIMI flow are well-known predictors of no-reflow post-PCI in STEMI patients1720. Direct stenting is a modifiable risk factor that should be tested in prospective randomised trials. However, there is a theoretical risk of stent undersizing, as reduced flow may lead to vessel size underestimation21. Intracoronary imaging may be helpful to identify coronary anatomy at high risk of no-reflow and to mitigate the risk of undersizing by allowing optimal size expansion and apposition2223.
Limitations
The present study has several limitations. First, the post hoc subgroup analyses should be considered hypothesis-generating. Second, as direct stenting was a post-randomisation variable, thrombectomy may have influenced the decision or the ability to use a direct-stenting strategy. It is possible that lower-risk patients with lower thrombus burdens may explain some of the association between direct stenting and no-reflow. Third, while our study sample is relatively large for a core laboratory- and physician-adjudicated clinical endpoint study, we had low power to detect differences for the secondary endpoints. However, the low interrater agreement suggests that blinded angiographic core laboratory analyses are essential, as operators may misclassify no-reflow. Finally, we did not have imaging studies correlating no-reflow status to infarct size or left ventricular dysfunction.
Conclusions
The present TOTAL angiographic core laboratory analysis demonstrates that thrombectomy did not reduce no-reflow in all patients but may be synergistic with direct stenting. No-reflow is associated with an increased risk of cardiovascular mortality, recurrent MI, cardiogenic shock, or NYHA Class IV heart failure. Contemporary randomised controlled trials are warranted to better assess the association between direct stenting and no-reflow.
Impact on daily practice
No-reflow management in ST-segment elevation myocardial infarction patients undergoing primary percutaneous coronary intervention is poorly defined. We performed a post hoc analysis of the TOTAL Trial angiographic core laboratory data (n=1,800), demonstrating that while thrombectomy did not reduce no-reflow in all patients, it may have a synergistic effect with direct stenting to decrease no-reflow. No-reflow is associated with an increased risk of cardiovascular mortality, recurrent myocardial infarction, cardiogenic shock, or NYHA Class IV heart failure.
Acknowledgments
The authors would like to acknowledge the diligent work of Mrs Fei Yuan and Peggy Gao for assisting with the statistical analyses.
Funding
Medtronic and the Canadian Institutes of Health Research supported the original TOTAL Trial.
Conflict of interest statement
C.B. Overgaard reports receiving consulting fees from Boston Scientific. J.A. Cairns reports receiving research grants from Medtronic, AstraZeneca, Boston Scientific, and Edwards Lifesciences; consulting fees from Abbott Vascular; and receipt of equipment and materials from AstraZeneca. S. Jolly reports receiving grants from Boston Scientific and honoraria from Penumbra. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

