
Abstract
Aims: The aim of this study was to ascertain whether a minimalist immediate mechanical intervention (MIMI) aiming to restore an optimal Thrombolysis In Myocardial Infarction (TIMI) flow in the culprit artery, followed ≥7 days later by a second percutaneous coronary intervention with intentional stenting, is safe in patients with ST-segment elevation myocardial infarction and large thrombotic burden.
Methods and results: SUPER-MIMI was a prospective, observational trial conducted between January 2014 and April 2015 in 14 French centres. A total of 155 patients were enrolled. The pharmacological therapy was left to the operator’s discretion. Eighty-one patients (52.3%) had glycoprotein IIb/IIIa inhibitors (GPI) initiated before the end of the first procedure. The median (interquartile range [IQR]) delay between the two procedures was eight (seven to 12) days. Infarct-related artery reocclusion between the two procedures (primary endpoint) occurred in two patients (1.3%), neither of whom received GPI treatment. TIMI flow was maintained or improved between the end of the first procedure and the beginning of the second procedure in all patients. Thrombotic burden and stenosis severity diminished significantly between the two procedures. Stents were ultimately implanted in 97 patients (62.6%).
Conclusions: Deferred stenting (≥7 days) in patients with a high thrombus burden was safe on a background of GPI therapy.
Abbreviations
BARC: Bleeding Academic Research Consortium
GPI: glycoprotein IIb/IIIa inhibitor(s)
IQR: interquartile range
MIMI: minimalist immediate mechanical intervention
PCI: percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
Introduction
Primary percutaneous coronary intervention (PCI) is the preferred reperfusion strategy in acute ST-segment elevation myocardial infarction (STEMI), the main objective being to relieve myocardial ischaemia by restoring epicardial coronary flow and myocardial tissue reperfusion1. However, up to two thirds of STEMI patients treated with primary PCI and stent implantation have suboptimal myocardial reperfusion, with undesirable short- and long-term consequences2. The pathological phenomenon associated with myocardial injury may occur secondary to downstream microvascular embolisation of thrombotic/atheromatous debris during PCI1.
To minimise injury in such a friable, emboligenic terrain, the “deferred/delayed stenting” concept was born: a first PCI aiming to restore the best possible Thrombolysis In Myocardial Infarction (TIMI) flow in the culprit artery using minimal intervention (none if the initial flow is TIMI 3), followed by a second intervention (hours/days later) to stent any residual lesion in a more stable environment. Initial studies have suggested favourable outcomes with delayed stenting regarding the rate of procedural success3, six-month left ventricular ejection fraction values4,5, and rates of major adverse cardiac events5-7. However, the randomised minimalist immediate mechanical intervention (MIMI) study8, which evaluated whether delayed (24-48 hours) versus immediate stenting improved myocardial reperfusion in a non-selected STEMI population treated with primary PCI, failed to show a reduction in microvascular obstruction with delayed stenting.
We hypothesised that delayed stenting would be more beneficial in patients with a high thrombus burden and a delay between the procedures of >2 days. Indeed, Sianos et al9 emphasised the influence of initial coronary thrombotic load on myocardial reperfusion in primary PCI. Moreover, Souteyrand et al10 demonstrated progressive thrombus regression during the first seven days after the acute event. It is therefore possible that a longer delay, to achieve optimal thrombus regression, would be beneficial before stent implantation. However, the benefit of a longer delay must be balanced against the risk of culprit artery reocclusion, although this would be low with the use of potent antiplatelet regimens. Therefore, this study was conducted primarily to demonstrate the safety of MIMI followed by a second PCI with the intention of culprit-lesion stenting after ≥7 days in patients with STEMI and a large thrombotic load.
Methods
SUPER-MIMI was a multicentre, prospective, observational trial performed in accordance with the Declaration of Helsinki and French laws. The protocol was approved by the national ethics committee (Commission Nationale de l’Informatique et des Libertés; authorisation number DR-2014-335). Patients provided oral informed consent.
Eligible adults (≥18 years) met the following three criteria at first coronary angiography: symptoms consistent with myocardial ischaemia and persistent (>30 minutes) ST-segment elevation ≥1 mm in ≥2 contiguous limb leads or >2 mm in ≥2 precordial leads on electrocardiogram, intended for primary, rescue (secondary to inefficient pharmacological reperfusion), or routine PCI <48 hours after successful pharmacological or spontaneous reperfusion; optimal epicardial flow in the culprit artery (assessed by the operator), either spontaneously or after minimal coronary manipulation using the thinnest tool/device (e.g., wire passage, thromboaspiration, or small diameter [≤2 mm] balloon); and indication for coronary stenting and a sufficiently important thrombotic burden for the operator to opt for delaying stent implantation for ≥7 days.
Pharmacological therapy - including glycoprotein IIb/IIIa inhibitors (GPI) - and the decision to postpone stent implantation based on the level of thrombotic burden were at the operator’s discretion. The main exclusion criteria were pre-hospital cardiac arrest, life expectancy <30 days, and indication for revascularisation by coronary artery bypass graft at the time of first coronary angiography. Patients selected for deferred stenting, but within seven days, were not included even if the second procedure was performed ≥7 days after the first.
Patients received pharmacological treatment according to current recommendations1. Between the two procedures, patients received anticoagulant treatment. Patients were divided into two groups according to GPI use for a post hoc analysis.
Data were collected and recorded in electronic case report forms. Distant monitoring ensured consistency. Two operators not involved in the study reviewed and analysed the angiograms: they independently quantified degree of stenosis in the culprit artery, thrombus burden, rate of no/slow flow, and distal embolisation related to the first procedure. They also independently confirmed data from case report forms for culprit artery identification, TIMI flow grade, the specific tools used to restore optimal coronary flow at the first procedure, and the final percutaneous treatment strategy (presence/absence of stent implantation) at the second procedure.
The primary endpoint was the rate of culprit artery reocclusion between the two procedures, defined as: (1) recurrence of symptoms typical of myocardial ischaemia (accompanied by ST-segment elevation in the same electrocardiogram leads as the initial event) leading to an urgent coronary angiogram that showed, with respect to the initial culprit artery, a TIMI flow at the beginning of the second procedure inferior to the TIMI flow at the end of the first procedure; or (2) unexplained sudden cardiac death between the two procedures. Secondary endpoints were: evolution of TIMI flow grade (independent of symptoms) between the procedures, thrombotic burden, degree of stenosis in the culprit artery, and the rate of stenting at the second procedure. Thrombus burden was evaluated angiographically using Gibson grades. We evaluated initial thrombotic burden after at least a TIMI 1 flow was obtained in the culprit artery, thereby resulting in a maximal Gibson grade of 4. Periprocedural bleeding complications were quantified using Bleeding Academic Research Consortium (BARC) definitions11. Periprocedural myocardial infarction was defined as a ≥5-fold increase in troponin between the beginning and end of the procedure; contrast-induced nephropathy was defined as an increase in creatinine >0.5 mg/dL or >25% from baseline.
STATISTICAL ANALYSIS
Continuous variables are expressed as medians (interquartile ranges [IQRs]), categorical variables as numbers (percentages). To compare thrombotic burden and degree of culprit artery stenosis, we used tests for paired samples, the Wilcoxon test for stenosis, and the Kendall test for Gibson score. To compare the proportions of patients with stenosis ≥50%, we used tests adapted to paired samples (McNemar test). A p-value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS, Version 22 (IBM Corp, Armonk, NY, USA).
Results
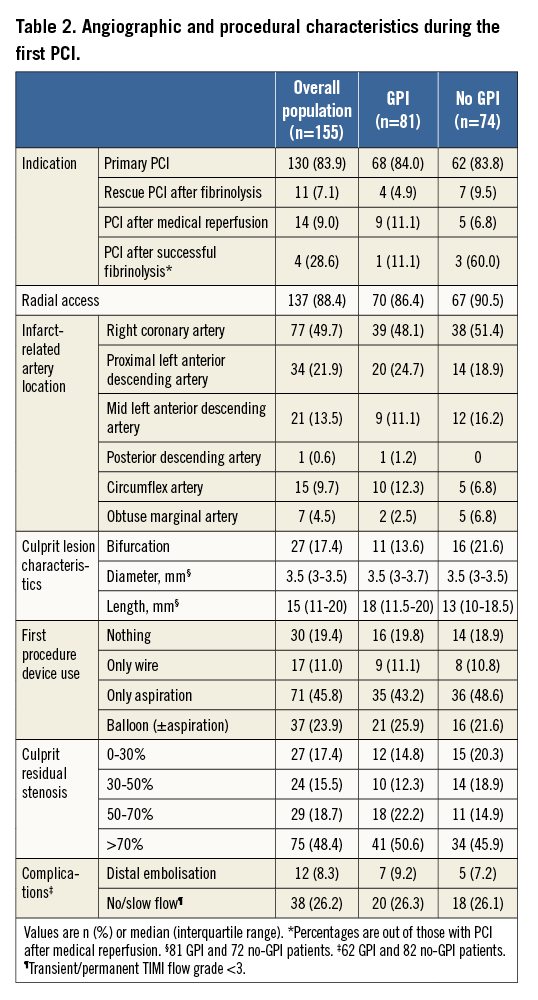
Between January 2014 and April 2015, from 4,410 patients admitted for acute STEMI treatment in 14 centres, 155 patients (3.5%) met the study criteria and were enrolled (Table 1).
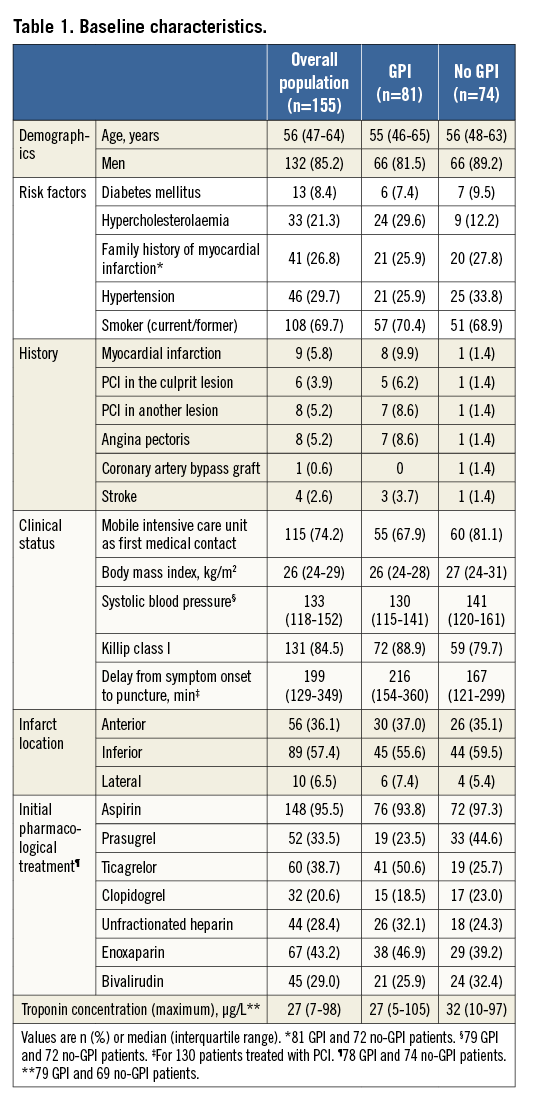
At the first PCI, most patients were admitted for primary PCI (Table 2). After MIMI (no intervention, guidewire, thromboaspiration, or balloon angioplasty), nearly all patients achieved TIMI flow 2-3 (Figure 1). Eighty-one patients (52.3%) had GPI initiated before the end of the procedure. Median residual stenosis was 70% (IQR 50-85%), and the rates of distal embolisation and no/slow flow were 8.3% and 26.2%, respectively (Table 2).
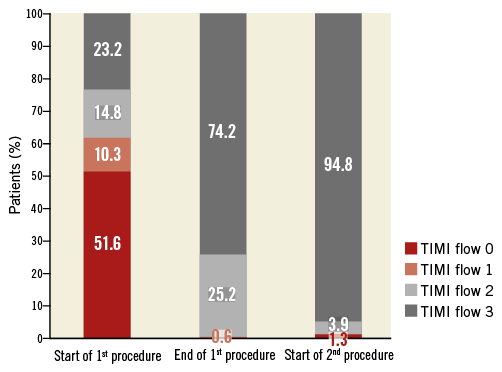
Figure 1. Evolution of TIMI flow grade in the infarct-related artery at the start and end of the first procedure and the start of the second procedure. TIMI: Thrombolysis In Myocardial Infarction
The median (IQR) delay between the two procedures was eight (seven to 12) days (Figure 2). The second procedure was performed ≤6 days after the first in 16.1% of patients for logistical reasons (n=22), reocclusion of the culprit artery (n=2), and recurrent angina leading to coronary artery bypass grafting (n=1). Two patients (1.3%) had culprit artery reocclusion between the two procedures 15 and 30 minutes after the first procedure (primary endpoint). Neither had received GPI. The first patient was a 56-year-old man admitted for coronary angiography 100 minutes after the clinical onset of an inferior STEMI complicated with a third-degree atrioventricular block. Pre-hospital treatment consisted of prasugrel, bivalirudin, and intravenous aspirin, noting that the oral medication administration was disturbed by vomiting. Coronary angiography showed a total thrombotic occlusion of the right coronary artery, without other significant lesions in the coronary tree. Thromboaspiration restored a TIMI 3 flow grade with a 75% thrombotic residual stenosis. GPI treatment was started 5 minutes after the first procedure, but 10 minutes later the patient suffered a recurrence of chest pain and ST-segment re-elevation in the inferior leads that justified a second coronary angiogram which showed a thrombotic reocclusion (TIMI 0) of the right coronary artery. Treatment consisted of stent implantation, and there was a good procedural result. The second patient was a 59-year-old man admitted for anterior STEMI 180 minutes after symptom onset. He was treated before the primary PCI with aspirin, prasugrel, and enoxaparin. Coronary angiography showed a long thrombotic stenosis of 70% in the left anterior descending artery with a TIMI 3 flow and other significant, but stable, stenoses in the left circumflex and right coronary arteries. Thromboaspiration achieved a residual stenosis of 50% with normal coronary flow. Thirty minutes after the procedure, the symptoms reoccurred, accompanied by a new anterior ST-segment elevation. Coronary angiography showed a thrombotic reocclusion of the proximal left anterior descending artery (TIMI 0) that was treated by thromboaspiration, stent implantation, and GPI. The procedural result and follow-up were favourable.
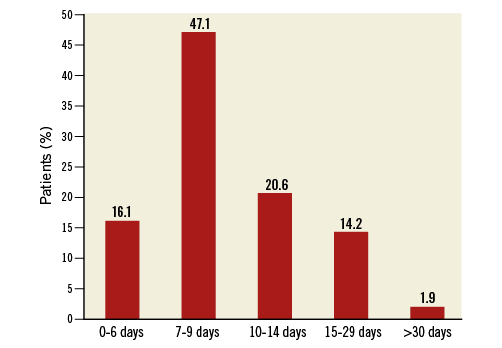
Figure 2. Delay between the two procedures.
Between the end of the first procedure and the beginning of the second, TIMI flow in the infarct-related artery improved overall (Figure 1), and did not worsen in any patient. The median (IQR) Gibson score decreased from 4 (0-7) at the end of the first procedure to 2 (1-6) at the beginning of the second (p<0.01). Figure 3 illustrates thrombus evolution between the two procedures. The degree of culprit artery stenosis diminished significantly between the procedures (Figure 4). At the end of the first PCI, 84.0% of patients had a stenosis >50%; this reduced to 50.0% at the beginning of the second PCI (p<0.001).
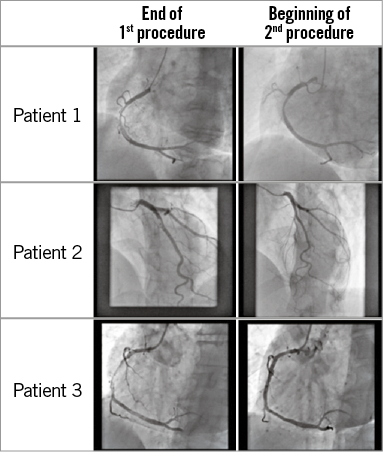
Figure 3. Examples of thrombus evolution between the procedures.
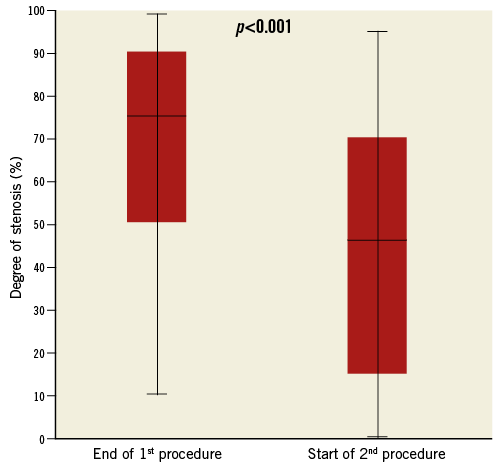
Figure 4. Evolution of the degree of stenosis between the procedures.
A stent was implanted in 97 patients (62.6%); the residual lesion was considered non-significant in 56 patients (36.1%) (Table 3). One patient was treated by coronary artery bypass grafting and one by deferred PCI (due to a large residual thrombotic burden at the time of the second procedure). Besides the two patients with reocclusion of the infarct-related artery, the overall rate of complications was 3.8% (6/155): three patients had a periprocedural myocardial infarction during the second procedure (excluding culprit artery reocclusions); two had rectorrhagia (Bleeding Academic Research Consortium 3a) (one patient [on GPI] bled 48 hours after the second procedure; the other [no GPI therapy] bled immediately after the first PCI); and one developed transient contrast-induced nephropathy after the first PCI (creatinine before 146 µM/L; after 196 µM/L) which was successfully controlled with adequate hydration. No deaths were recorded between the first and second procedures.
Discussion
The SUPER-MIMI study demonstrated the safety of a new PCI strategy in STEMI patients with suspected large culprit artery thrombotic burden: the strategy comprised a minimalist immediate mechanical intervention to restore optimal blood flow in the responsible artery followed by a second PCI to stent the culprit lesion ≥7 days later.
Only 2/155 patients had culprit artery reocclusions (neither received GPI) between the two procedures, both of which occurred soon after the first procedure. Our findings are similar to the rate of culprit artery reocclusion in non-selected STEMI populations treated with immediate stenting (0.6-1.7%)12-14. However, our patients had high thrombotic burden, and stenting in such patients carries a higher risk of acute stent thrombosis than in non-selected STEMI patients.
Three randomised studies have investigated a deferred strategy in STEMI, with dissimilar results. DEFER-STEMI15 demonstrated a lower rate of no/slow reflow with deferred (four to 16 hours) versus immediate stenting (5.9% vs. 28.6%; p=0.005; primary outcome) among patients with ≥1 risk factor for no reflow. The rate of culprit artery reocclusion in the deferred group was 3.8%. However, MIMI8 found no significant difference in microvascular obstruction between deferred (24-48 hours) and immediate stenting (4.0% vs. 1.9%; p=0.051; primary endpoint) and no culprit artery reocclusion occurred in the former. DANAMI 3-DEFER16 reported no difference in the composite of all-cause mortality, hospital admission for heart failure, recurrent infarction and any unplanned revascularisation of a target vessel in two years of follow-up between >24-hour deferred (median [IQR] delay of three1-4 days) and immediate stenting (17% vs. 18%; p=0.92; primary endpoint); the frequency of infarct-related artery reocclusion was 1.3% in the deferred group (secondary endpoint).
A meta-analysis17 of six observational studies comparing immediate versus delayed stenting in patients with acute coronary syndromes reported no culprit artery reocclusions between the procedures with delayed stenting among those with STEMI (n=175). Similar results were recently reported by Souteyrand et al10, who recorded no culprit artery reocclusion with a mean time of five days between the initial and second procedure (n=101).
Regarding the safety of the SUPER-MIMI strategy, culprit artery reocclusion (in our study and DEFER-STEMI15) occurred early after the first PCI (<2 hours), facilitating immediate treatment. Our results may indicate the importance of early adjunctive GPI therapy (started before the end of the first procedure), as neither patient with a reocclusion received GPI treatment. In MIMI8, patients were treated with GPI before randomisation. In DEFER-STEMI15, GPI were started after randomisation, during or immediately after the first procedure, leading to a short vulnerable window. In DANAMI 3-DEFER16, GPI were only used in 35% of patients in the deferred stenting group. Two situations may be important regarding GPI therapy. Firstly, GPI would increase the risk of bleeding after fibrinolysis, and this could be critical when deferred stenting is an option. However, an observational study18 showed few bleeding complications in such patients. In SUPER-MIMI, GPI therapy was administered in 5/15 patients (33.3%) after fibrinolytic therapy, with no major bleedings. Secondly, one patient who met the primary endpoint received bivalirudin, which has been associated with acute stent thrombosis19. Therefore, the place of GPI therapy in this situation could be further consolidated.
In our study, five patients with low thrombotic burden (Gibson score <3) at the end of the first PCI were enrolled. This is because thrombus burden quantification in the primary PCI setting is challenging, and the proportions of thrombotic and atherosclerotic components in the residual lesion may be questionable. However, high thrombus burden can be suspected in the presence of long, complex, high-volume, compact lesions. Therefore, the Gibson score may not be the most accurate method for thrombus burden quantification in “high thrombus suspected” lesions. After the specified time window between the initial angiographic evaluation and the final therapeutic decision, a significant reduction in the degree of stenosis was reported. This positive evolution, mainly due to thrombus resolution, is one of the possible benefits of the SUPER-MIMI strategy, leading to the avoidance of stenting in 36.1% of cases.
Nevertheless, prolongation of hospital stay may jeopardise the cost of strategies such as SUPER-MIMI. The cost-effectiveness of this strategy and the subsequent clinical evolution of patients included in the study have yet to be investigated.
Limitations
For logistical reasons, 25 patients (16.1%) had <7 days between the two procedures (including both those with reocclusion), but remained in the intent-to-treat population. GPI were not routinely used, despite a presumed large thrombus burden. This may have been due to high bleeding risk, a long time between symptom onset and PCI, or a low amount of jeopardised myocardium. In 25.8% of patients, TIMI 3 flow was not achieved at the end of the first procedure. This was because the operator considered that coronary flow could not be improved by further percutaneous manipulation (including stent implantation) in the absence of continuous myocardial ischaemia, implying the possible involvement of the collateral coronary network. This analysis does not include long-term follow-up data; once available, these data will be the subject of a future article.
Conclusions
These observational data suggest that a strategy of deferred stenting (≥7 days) in patients with a high thrombus burden appears safe, whereas delayed stenting without GPI may be questionable. A substantial proportion of patients did not require stent implantation as the final therapeutic strategy. These findings require confirmation in larger randomised trials.
| Impact on daily practice Intentionally delaying stenting procedures for ≥7 days (after MIMI) in STEMI patients with high thrombotic burden appears safe and reduces the need for stent insertion. However, patients should probably be given GPI to reduce the risk of infarct-related artery reocclusion. |
Acknowledgements
The authors wish to thank Cecile Ricard for statistical analysis, Mihaela Rata and Loic Lamboley for data analysis, and MedLink Healthcare Communications for editorial support (funded by the authors).
Funding
Correvio International Sarl, Switzerland (main sponsor), Medtronic, Boston Scientific, Abbott, Cordis, St. Jude Medical, Biotronik, Terumo and AstraZeneca.
Conflict of interest statement
M. Abdellaoui has received research grants from Biosensors International Group, Edwards Lifesciences, and Boston Scientific. G. Souteyrand is a consultant for St. Jude Medical, Abbott, Terumo Corporation, and Medtronic. L. Belle has received research grants from Novartis, AstraZeneca, Lilly, Sanofi, Correvio International, Medtronic, Biotronik, Abbott, Boston Scientific, CeloNova, Terumo Corporation, and St. Jude Medical. The other authors have no conflicts of interest to declare.

