
Abstract
Primary percutaneous coronary intervention (PPCI) has become the mainstay of reperfusion therapy in patients with ST-segment elevation myocardial infarction (STEMI). Despite timely reperfusion by PPCI and restoration of epicardial blood flow in up to 95% of patients, tissue reperfusion remains suboptimal in a sizeable proportion of patients with STEMI. Over the years mechanical and pharmacological strategies to enhance myocardial salvage during PPCI have been developed and used in patients with STEMI. The most common mechanical strategies used in the setting of PPCI include: coronary stenting, direct stenting, mesh-covered stents, self-expanding stents, deferred stenting, thrombectomy, distal protection devices, intra-aortic balloon pumping, left ventricular assist devices and ischaemic conditioning. These strategies are thought to enhance myocardial salvage via improving acute procedural success, attenuation of distal embolisation, microvascular obstruction and reperfusion injury, and providing haemodynamic support. Coronary (direct) stenting is almost the default approach of reperfusion during PPCI procedures. Evidence on the use of mesh-covered stents, self-expanding stents, deferred stenting or left ventricular assist devices is scant and their use in the setting of PPCI remains limited. Mechanical thrombectomy, distal protection devices or routine intra-aortic balloon counterpulsation seem to offer no clinical benefit when used in the setting of PPCI. Although manual aspiration may improve indices of tissue reperfusion, recent research showed no clinical benefit of routine use of this strategy in patients with STEMI undergoing PPCI. Ischaemic conditioning, although promising, remains at an investigational stage and needs further research.
Introduction
Over the last few decades, considerable efforts have been made at societal and medical community level to improve the therapy of patients with ST-segment elevation myocardial infarction (STEMI) by working in three fields: 1) increased availability of intervention centres capable of performing primary percutaneous coronary intervention (PPCI) and building of triage and transfer systems of care to provide timely access to reperfusion in STEMI patients; 2) improvement of the PPCI equipment including new generations of coronary stents and their delivery systems and adjunct pharmacologic therapy (antithrombotic/anticoagulant drugs); and 3) development and evaluation of strategies to enhance myocardial salvage during PPCI procedures via optimising acute procedural success, attenuation of distal embolisation, microvascular obstruction and reperfusion injury, and providing haemodynamic support1. Pharmacological strategies to promote myocardial salvage during PPCI have recently been reviewed1. The focus of this review is to summarise mechanical strategies that are used to enhance myocardial salvage during PPCI procedures (Figure 1). The use of these strategies in cardiogenic shock is not covered.
Strategies to reduce distal embolisation
Although PPCI restores epicardial blood flow in up to 95% of patients with STEMI, tissue reperfusion often remains suboptimal, mostly due to persistent (micro)vascular obstruction leading to increased infarct size (IS), adverse left ventricular remodelling and increased mortality. Among various mechanisms suggested to explain microvascular obstruction and no-reflow following PPCI, distal embolisation of thrombotic and/or atheromatous debris is believed to play an important role in the genesis of this condition and subsequent adverse clinical outcome2. In patients with STEMI undergoing PPCI, distal emboli have been visualised with a Doppler guidewire3, and visible debris has been retrieved (in distal protection filters) in 73% of patients with STEMI undergoing PPCI4. Distal embolisation has been implicated in the suboptimal tissue reperfusion and poor outcome after PPCI5,6. A recent study showed that distal embolisation occurred in 11% of patients with STEMI treated with conventional PPCI and that its occurrence increased the risk of heart failure7. Over the years, various mechanical strategies aiming at reduction of distal embolisation during PPCI have been developed (Figure 1).
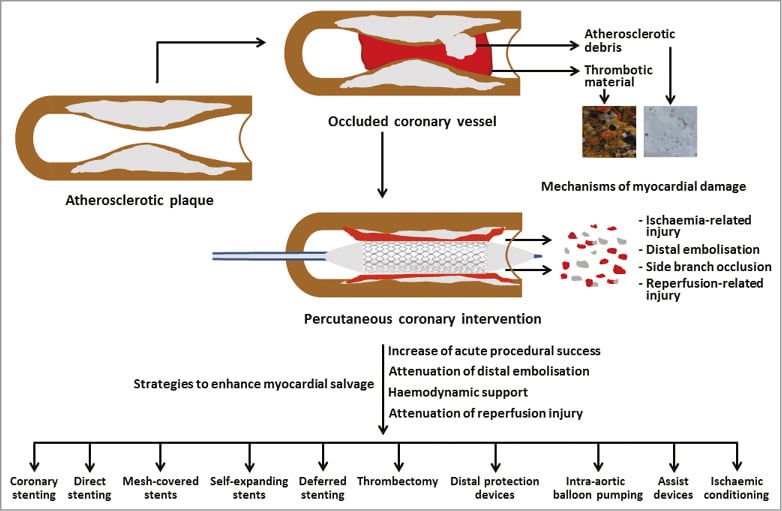
Figure 1. Mechanical strategies to enhance myocardial salvage during PPCI in patients with STEMI.
CORONARY STENTING
In the early days of mechanical reperfusion for STEMI, plain balloon angioplasty was the mainstay of therapy. Coronary stenting in the setting of PPCI for STEMI was considered contraindicated due to concerns that implantation of a metallic structure within the thrombogenic environment in the infarct-related artery would predispose to acute stent thrombosis and coronary reocclusion. The use of balloon angioplasty alone was associated with suboptimal results, mostly related to recurrent ischaemia and reocclusion within the first days or weeks after the procedure and a high incidence of restenosis. The Stent versus Thrombolysis for Occluded Coronary Arteries in Patients with Acute Myocardial Infarction (STOPAMI) trial showed that coronary stenting plus abciximab is safe and leads to a greater degree of myocardial salvage and a better clinical outcome than fibrinolysis with a tissue plasminogen activator. Final IS (estimated by repeat scintigraphic studies) was 14.3% in the group with stenting and 19.4% of the left ventricle in the group with thrombolysis (p=0.02); the salvage index (proportion of initial area at risk salvaged by reperfusion) was 57% in the stent group vs. 26% in the thrombolysis group (p<0.001), and the cumulative six-month incidence of death, myocardial infarction or stroke was lower among patients treated with stenting (8.5% vs. 23.2%; p=0.02)8. The STOPAMI trial offered mechanistic information that explains the superiority of stenting over fibrinolysis in patients with STEMI. A meta-analysis that included 13 randomised trials with 6,922 patients showed that stenting significantly reduced the one-year incidence of repeat revascularisation (11.3% vs. 18.4%) but had no effect on reinfarction (3.7% vs. 3.9%) or mortality (5.1% vs. 5.2%) compared with balloon angioplasty9. The Primary Angioplasty in Myocardial Infarction (PAMI) trial showed better angiographic results and a sustained benefit in mortality at one and five years with stenting compared with balloon angioplasty10. Mechanistically, coronary stents achieve better angiographic results (less residual stenosis), fewer early ischaemic events because of the sealing of plaque rupture and dissection, and longer-term patency due to lessening of the elastic recoil and constrictive remodelling compared with balloon angioplasty alone. These studies and other evidence transformed coronary stenting from a feared therapeutic option to a default PPCI strategy in patients with STEMI.
The clinical experience of using bioresorbable scaffolds in patients with STEMI is limited. The randomised multicentre ABSORB-STEMI TROFI II trial assigned 191 patients with STEMI to receive an everolimus-eluting bioresorbable stent or a durable polymer everolimus-eluting metallic stent. The primary outcome was the six-month optical frequency domain imaging healing score. The study found that stenting of culprit lesions with the bioresorbable stent in the setting of STEMI resulted in a nearly complete arterial healing which was comparable with that of a durable polymer metallic stent at six months. The procedural and clinical results were encouraging11. However, in general, there are concerns with the current generation(s) of bioresorbable scaffolds related to strut thickness, poor deliverability and lack of radial strength requiring, for preference, predilatation12. The optimal duration of antithrombotic therapy after bioresorbable scaffold implantation is not clear. To what extent these limitations will impact on the use of these devices in patients with STEMI remains to be seen.
DIRECT STENTING
Evidence in favour of direct stenting (stenting without predilation) in patients with STEMI comes from one randomised study, observational studies or subgroup analyses. Loubeyre et al13 randomised 206 patients with STEMI to direct stenting or stent implantation after balloon predilation. The composite angiographic (corrected Thrombolysis In Myocardial Infarction [TIMI] frame count, slow-flow/no-reflow or distal embolisation) endpoint (11.7% vs. 26.9%; p=0.01) and ST-segment resolution (79.8% vs. 61.9%; p=0.01) were better among patients randomised to direct stenting than among those randomised to stent implantation after predilation13. In a cohort of 423 consecutive patients with STEMI undergoing PPCI with stenting (110 patients with direct stenting), direct stenting reduced the incidence of angiographic no-reflow (5.5% vs. 12.0%; p=0.04) and one-month mortality (1.0% vs. 8.0%; p=0.008) compared with stenting after predilation14. In the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI), direct stenting (n=698) compared with conventional stenting after predilation (n=1,830) was associated with better ST-segment resolution at 60 minutes after the procedure (median: 74.8% vs. 68.9%; p=0.01) and lower one-year rates of all-cause mortality (1.6% vs. 3.8%; p=0.01) and stroke (0.3% vs. 1.1%; p=0.049)15. In a recent UK study that included 1,562 unselected, contemporary patients undergoing PPCI for STEMI (489 patients with direct stenting), direct stenting was associated with better 30-day (2.04% vs. 4.66%; p=0.01) and one-year (3.27% vs. 8.48%; p<0.001) mortality compared with stenting after predilation16.
The EUROTRANSFER Registry that included 1,419 patients showed that direct stenting (n=276) was superior to stenting after predilation in terms of post-procedural TIMI flow grade of 3 (94.9% vs. 91.5%; p=0.02), no-reflow (1.4% vs. 3.4%; p=0.035), ST-segment resolution of >50% (86.2% vs. 76.3%; p=0.016) and one-year mortality (2.9% vs. 6.5%; p=0.047 after adjustment for propensity score)17. Direct stenting may be advantageous over stenting after predilation in several aspects including the use of fewer and shorter stents, shorter fluoroscopy time and less use of contrast media and reduced microvascular dysfunction/obstruction and no-reflow by reduced distal embolisation. Potential disadvantages of direct stenting may include: failure to reach and/or to cross the lesion, stent loss, erroneous estimation of stent length, difficulty with stent positioning (especially in case of persistent TIMI flow 0-1), underexpansion of the stent in an undilatable (i.e., calcified) lesion and stent undersizing due to underestimation of vessel diameter because of reduced flow18. Notwithstanding these disadvantages, direct stenting is considered almost as a default strategy during PPCI. The combination of direct stenting with aspiration thrombectomy –hailed for the advantages of direct stenting in prior studies– has recently been questioned in the light of suboptimal results with aspiration thrombectomy.
MESH-COVERED STENTS
The MGuard™ mesh-covered stent (InspireMD, Boston, MA, USA), a bare metal stent with a polyethylene terephthalate MicroNet™ mesh covering, has been designed to prevent distal embolisation by trapping and excluding embolism-prone material at the level of the culprit lesion in patients with STEMI. After small studies testing the feasibility and safety of the MGuard stent19, the Safety and Efficacy Study of MGuard Stent After a Heart Attack (MASTER) trial tested the efficacy of the stent in the setting of PPCI. The study randomly assigned 433 patients with STEMI presenting within 12 hours to receive the MGuard stent or a commercially available bare metal or drug-eluting stent. The primary endpoint (ST-segment resolution ≥70%, 60-90 minutes after procedure) was significantly improved in patients randomised to the MGuard stent compared to control patients (57.8% vs. 44.7%; p=0.008). TIMI flow grade 3 was more frequent among patients who received the MGuard stent (91.7% vs. 82.9%; p=0.006). In 59 patients (30 patients assigned to the MGuard stent), cardiac magnetic resonance (CMR) was performed at three to five days; it did not show a significant difference in the IS expressed as mass (median: 17.1 g vs. 22.3 g; p=0.27) or percentage of the left ventricular mass (median: 13.3% vs. 16.6%; p=0.48) between patients assigned to the MGuard stent or controls. Mortality (0% vs. 1.9%; p=0.06) and major adverse cardiac events at 30 days (1.8% vs. 2.3%; p=0.75) did not differ significantly between patients assigned to the MGuard stent or controls20. At one year, the incidence of major adverse cardiac events (all-cause death, reinfarction, or ischaemia-driven target lesion revascularisation) was higher among patients with the MGuard stent (9.1% vs. 3.3%, p=0.02), driven by more frequent ischaemia-driven target lesion revascularisation compared to patients with conventional stenting. One-year mortality tended to be lower with the MGuard stent (1.0% vs. 3.3%; p=0.09). The binary restenosis rate (assessed in 38 patients with the MGuard stent) on 13-month angiography was 31.6%21. The MGuard stent may be useful to prevent distal embolisation in patients with STEMI and high thrombus burden22. Notwithstanding these results, the use of mesh-covered stents in patients with STEMI remains limited. Mesh-covered stents should be avoided in bifurcational interventions. The development of drug-eluting mesh-covered stents may enhance the efficacy of this technology.
SELF-EXPANDING STENTS
The presence of thrombus and epicardial vasoconstriction may lead to underestimation of the vessel size, which increases the risk of stent undersizing – a well-known factor for stent thrombosis23,24. The ability of the self-expanding stents to grow gradually in size may allow stent deployment at lower pressures, which may lead to less local trauma. Less local trauma could result in less plaque disruption and less distal embolisation of thrombotic-atherosclerotic debris25,26. A feasibility study of 25 patients with STEMI showed that use of the STENTYS (STENTYS S.A., Paris, France) self-expanding stent is safe and feasible in these patients. Angiography and intravascular ultrasound or optical coherence tomography were performed immediately after stent deployment, after three days and at six months. The imaging studies showed that, three days after the procedure, the stent expanded to the same extent as the epicardial vasodilatation and appeared completely apposed to the vessel wall. No death, reinfarction or stent thrombosis occurred over six months of follow-up27. Despite these results, the experience with the use of self-expanding stents in the setting of PPCI remains rather limited. Furthermore, concerns have been raised on the optimal stent/vessel ratio, continuation of self-expansion after stent deployment predisposing for plaque prolapse and arrest of self-expansion in calcified lesions28.
DEFERRED STENTING
Deferred stenting refers to a two-step strategy of initial reperfusion by balloon angioplasty (or thrombus removal) followed by stent implantation hours (or days) after the initial procedure. A deferred stenting strategy has also been pursued following initial minimal interventions (small size balloons to avoid both large dissection and distal embolisation sufficient to restore flow in the infarct-related artery which is sustained by maximised antithrombotic therapy)29 or after spontaneous reperfusion with optimal TIMI flow and ST-segment recovery30. Observational studies have shown that a deferred stenting strategy is safe in the majority of patients with STEMI31. A 2013 meta-analysis of patients with STEMI and non-STEMI concluded that delayed stenting is associated with better angiographic outcomes compared with immediate stenting32. The Deferred Stenting Versus Immediate Stenting to Prevent No- or Slow-Reflow in Acute ST-Segment Elevation Myocardial Infarction (DEFER-STEMI) trial randomised 101 patients with STEMI with ≥1 risk factors for no-reflow to deferred stenting (four to 16 hours after initial reperfusion) or immediate stenting33. The primary endpoint was the incidence of angiographic no-reflow/slow-reflow. Aspiration thrombectomy was performed in 88.5% and 85.7% of the patients undergoing deferred or immediate stenting, respectively. In the deferred stenting group, the median time to second procedure was nine hours. The primary endpoint (6% vs. 29%; p=0.006) and the frequency of no-reflow (2% vs. 14%; p=0.052) were lower in patients assigned to deferred stenting. In the two-day CMR, microvascular obstruction was present in 47.9% of patients with deferred stenting and 61.7% of patients with immediate stenting (p=0.155). In the six-month CMR, myocardial salvage (median: 19.7% vs. 14.7% of the left ventricular mass; p=0.027) and salvage index (median: 68% vs. 56%; p=0.031) were greater in the deferred stenting group. However, the IS did not differ significantly at six months after the procedure in the deferred vs. immediate stenting groups (median: 9.0% vs. 14.3% of the left ventricle; p=0.181). Mechanistically, a strategy of deferred stenting may reduce distal embolisation of thrombotic and/or vasopressor material compared with a strategy of immediate stenting. Following an initial procedure of flow restoration, a progressive reduction of the thrombotic burden without causing distal microvascular obstruction has been observed34. Several randomised studies are being conducted to explore the benefits of delayed vs. immediate stenting: Optimising Infarct Size by Transforming Emergent Stenting Into an Elective Procedure Study (OPTIMASTRATEGY; NCT01462188), the DANish Study of Optimal Acute Treatment of Patients With ST-elevation Myocardial Infarction (DANAMI-3; NCT01435408), the Minimal Invasive Procedure for Myocardial Infarction (MIMI; NCT01360242) and the Primary Reperfusion Secondary Stenting Trial (PRIMACY; NCT01542385).
MECHANICAL AND ASPIRATION THROMBECTOMY
Thrombectomy devices have been used in the setting of PPCI to reduce the chance (or extent) of distal embolisation by removing thrombotic material from the occluded coronary arteries. Thrombus removal is enabled by mechanical or aspiration thrombectomy strategies. It is not recommended to perform mechanical thrombectomy in the setting of PPCI in patients with STEMI.
Earlier randomised trials of aspiration thrombectomy gave encouraging results in terms of improved clinical outcome by this strategy. The Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS) trial randomised 1,071 patients with STEMI to aspiration thrombectomy plus conventional PCI vs. PCI alone. Aspiration thrombectomy improved tissue reperfusion (blush grade 0-1, 17.1% vs. 26.3%, p<0.001) and complete (>70%) ST-segment resolution (56.6% vs. 44.2%; p<0.001) and was associated with a trend towards lower 30-day mortality (2.1% vs. 4.0%; p=0.07) compared with conventional PCI. One-year results of the TAPAS trial showed a significant reduction of cardiac (3.6% vs. 6.7%; p=0.02) and all-cause mortality (4.7% vs. 7.6%; p=0.042) by aspiration thrombectomy35. The Thrombectomy With Export Catheter in Infarct-Related Artery During Primary Percutaneous Coronary Intervention (EXPIRA) trial showed higher rates of blush grade ≥2 (88% vs. 60%; p=0.001) and ST-segment resolution >70% (64% vs. 39%; p=0.001) with aspiration thrombectomy compared with PCI alone36. In a group of 75 patients with anterior STEMI, microvascular obstruction - assessed by CMR - was less among patients with manual aspiration (31.5% vs. 72.9% of the patients; p=0.0005; or 1.7 g vs. 3.7 g; p=0.0003). In the acute phase, IS was not reduced by manual aspiration (mean: 13% vs. 14% of the left ventricle; p=0.60 or 14 g vs. 17 g; p=0.20). However, at three months IS was reduced only in the group with manual aspiration plus PCI.
The recent research in the field of manual thrombectomy in the setting of PPCI did not offer evidence on the beneficial effects of this strategy in patients with STEMI. The Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia (TASTE) trial did not show a benefit of manual aspiration thrombectomy compared to PPCI alone in terms of improved clinical outcome37. The study included 7,244 patients with STEMI undergoing PCI randomised to manual aspiration followed by PCI or to PCI only. The 30-day incidence of all-cause mortality (2.8% vs. 3.0%; p=0.63), hospitalisation for recurrent myocardial infarction (0.5% vs. 0.9%; p=0.09) and stent thrombosis (0.2% vs. 0.5%; p=0.06) did not differ significantly among patients treated with manual aspiration plus PCI or PCI only37. Notably, the rates of stroke and neurologic complications at the time of discharge did not differ in groups with or without manual aspiration (p=0.87). The outcome analysis at one year did not find any clinical benefit of manual aspiration irrespective of thrombus burden or coronary flow before PCI, ruling out any late benefit of this strategy in patients with STEMI38. The Trial of Routine Aspiration Thrombectomy with PCI versus PCI Alone in Patients with STEMI (TOTAL) study delivered another blow to the use of manual aspiration as an adjunct to PPCI39. The study randomised 10,732 patients with STEMI undergoing PPCI to a strategy of routine upfront manual thrombectomy vs. PCI alone. The primary outcome –a composite of cardiovascular death, recurrent myocardial infarction, cardiogenic shock, or New York Heart Association Class IV heart failure within 180 days– occurred in 6.9% of patients in the thrombectomy vs. 7.0% (p=0.86) in the PCI-alone group. Stroke within 30 days (secondary outcome) occurred in 0.7% of patients in the thrombectomy group vs. 0.3% of patients in the PCI-alone group (hazard ratio of 2.06; p=0.02). Apart from confirming the lack of efficacy of manual thrombotic aspiration, the TOTAL trial was important in offering evidence on the increased risk of neurological complications, potentially due to an increased risk of systemic embolism by the procedure in the setting of PPCI39.
The impact of aspiration thrombectomy on IS was assessed in a recent meta-analysis of seven studies with 950 patients. IS – estimated by CMR or single photon emission tomography – did not differ between the aspiration thrombectomy and PCI-only arms (17.1% vs. 17.3%; p=0.64). When the analysis was restricted to CMR studies only, again there was no difference in the IS between the study arms (p=0.23)40.
In the light of current research, current guidelines have downplayed the role of aspiration thrombectomy during PPCI by giving a class IIb recommendation (not well established value) for the use of selective or bail-out aspiration thrombectomy and a class III recommendation (no benefit) for the use of routine aspiration thrombectomy before PPCI41.
DISTAL PROTECTION DEVICES
The strategy of distal protection during PPCI consists in the deployment of devices (distal filters, distal occluders, proximal occluders or thrombus extraction devices) to restrict distal embolisation of debris dislodged from the culprit lesions at the time of PPCI. Distal protection devices have improved clinical outcome when used to treat stenotic lesions in bypass graft vessels42. Despite ample evidence that distal protection devices can be safely deployed and that they effectively retrieve debris, most of the clinical research on the efficacy of these devices in patients with STEMI has been disappointing. The Enhanced Myocardial Efficacy and Recovery by Aspiration of Liberated Debris (EMERALD) trial randomised 501 patients with STEMI presenting within six hours who underwent primary or rescue PCI to receive PCI with a balloon occlusion and aspiration distal microcirculatory protection system or angioplasty without distal protection. ST-segment resolution (>70%) measured 30 minutes after PCI and IS measured by technetium Tc 99m sestamibi imaging at five to 14 days were co-primary endpoints. Visible debris was retrieved from 73% of the patients. ST-segment resolution (63.3% vs. 61.9%; p=0.78), IS (median: 12.0% vs. 9.5% of the left ventricle; p=0.15) and the six-month composite endpoint of major adverse cardiac events (10.0% vs. 11.0%; p=0.66) did not differ among patients assigned to distal protection or not4. The Drug Elution and Distal Protection in ST-Elevation Myocardial Infarction (DEDICATION) trial randomised 626 patients with STEMI referred within 12 hours for PPCI to distal protection with a filterwire system (FilterWire EZ™; Boston Scientific, Marlborough, MA, USA) or conventional stenting without distal protection. The primary endpoint was complete ST-segment resolution measured by continuous ST-segment monitoring. Peak values of cardiac troponin T and creatine kinase myocardial band were used as estimates of IS. There was no significant difference in ST-segment resolution (76% vs. 72%; p=0.29), peak cardiac troponin T (4.8 µg/l vs. 5.0 µg/l; p=0.87) or peak creatine kinase myocardial band (185 µg/l vs. 184 µg/l; p=0.99) among patients assigned to distal protection or not. There was a trend towards a higher rate of major adverse cardiac and cerebral events at one month after PPCI in patients with distal protection (5.4% vs. 3.2%; p=0.17)43. Another randomised trial came to similar conclusions regarding the efficacy of distal protection devices to improve reperfusion or reduce IS in patients with STEMI44.
Reasons for the failure of distal protection devices to improve reperfusion in the setting of PPCI remain unknown. However, the embolisation caused by crossing the lesion with the device, impaired microcirculation by the device (non-embolic effects), dislodgement and embolisation of vasoconstrictor material not halted by the device, and failure to protect downstream side branches have been proposed as putative mechanisms. Distal protection devices are not recommended to be used in the setting of PPCI.
Strategies to provide haemodynamic support
INTRA-AORTIC BALLOON COUNTERPULSATION
The use of intra-aortic balloon counterpulsation is associated with immediate haemodynamic effects that lead to increased diastolic pressure, increased coronary perfusion pressure and reduced left ventricular afterload. All these effects are believed to be beneficial in patients with STEMI undergoing PPCI. It has been shown that intra-aortic balloon counterpulsation reduces the IS in an experimental setting45 and may prevent early infarct extension and ventricular remodelling in a clinical setting46. The impact of intra-aortic balloon counterpulsation on IS was investigated in the Counterpulsation to Reduce Infarct Size Pre-PCI for Acute Myocardial Infarction (CRISP-AMI) trial. The trial included 337 patients with anterior wall STEMI who were randomised to receive intra-aortic balloon pumping, initiated before PCI and continued for ≥12 hours, plus PCI or PCI alone. The primary outcome was IS measured by CMR performed three to five days after the procedure. IS was not significantly different between the patients in the intra-aortic balloon counterpulsation plus PCI group vs. the PCI-alone group (mean: 42.1% vs. 37.5% of the left ventricle; p=0.06). At 30 days, there was no significant difference between the groups regarding major vascular complications (p=0.09) or major bleeding and blood transfusion (p=0.49). The six-month mortality was 1.9% among patients assigned to intra-aortic balloon counterpulsation plus PCI and 5.2% among patients assigned to PCI alone (p=0.12)47. A recent meta-analysis of six trials with 1,054 patients (49.1% with intra-aortic balloon counterpulsation) showed that intra-aortic balloon counterpulsation did not reduce all-cause mortality (4.4% vs. 4.1%; p=0.80), congestive heart failure (17.1% vs. 18.0%; p=0.83) or reinfarction (5.3% vs. 7.7%; p=0.42). Intra-aortic balloon counterpulsation reduced recurrent ischaemia (3.6% vs. 20.3%; p<0.001) but it increased the risk of cerebrovascular accidents (2.0% vs. 0.3%; p=0.03) and bleeding (21.4% vs. 16.1%; p=0.02)48. Based on these data, the routine use of intra-aortic balloon counterpulsation in patients with STEMI does not seem to be justified.
ASSIST DEVICES
In analogy with intra-aortic balloon counterpulsation, left ventricular assist devices unload the left ventricle and when used in addition to reperfusion therapy may reduce IS and give the myocardium time to recuperate. These devices have mostly been used in patients with STEMI complicated by cardiogenic shock. The use of these devices in haemodynamically less compromised patients with STEMI is rather limited. The Academic Medical Center Mechanical support for Acute Congestive Heart failure in STEMI patients (AMC MACH) 2 study assessed the safety and feasibility of left ventricular unloading with the Impella® LP2.5 (Abiomed Europe GmbH, Aachen, Germany) in patients with first anterior STEMI presenting within the first six hours from symptom onset and without cardiogenic shock. Immediately after PCI, 10 patients received three days of Impella support and 10 concurrent patients received routine care including intra-aortic balloon counterpulsation if indicated. Impella insertion was successful in all cases. In the Impella group, the left ventricular ejection fraction improved from 28% at baseline to 37% at 3 days (p<0.05) and 41% at four months (p<0.05). Nevertheless, support for these results is limited due to the rather small number of patients and the non-randomised design of the study49.
Strategies to attenuate reperfusion injury
ISCHAEMIC CONDITIONING
Based on the results of experimental studies, it is assumed that nearly 50% of final IS is due to reperfusion injury, or myocardial injury following restoration of the blood flow in the infarct-related artery50. Ischaemic conditioning is a collective term that refers to an endogenous cardioprotection enabled by deliberate blood flow interruption in the infarct-related artery before coronary occlusion (ischaemic preconditioning), after coronary occlusion (ischaemic postconditioning) or an organ other than the heart (remote conditioning). Although ischaemic preconditioning was found to exert powerful effects against reperfusion injury and reduce IS, this approach is not practical in the setting of PPCI since it implies application of the stimuli prior to coronary occlusion which cannot be predicted in patients with STEMI.
Ischaemic postconditioning (transient episodes of deliberate ischaemia/reperfusion caused by repetitive inflation/deflation of an occluding balloon in the infarct-related artery) has been demonstrated to reduce IS by 44% in a canine model51. Mechanistically it is deemed that postconditioning activates cellular pro-survival pathways via various mediators (adenosine, nitric oxide, opioids, bradykinin or hypothetic peptides) leading to attenuation of reperfusion injury. Randomised studies gave conflicting results with regard to the impact of postconditioning on IS. A study of 50 patients with STEMI showed that postconditioning reduced IS and myocardial oedema assessed by CMR (mean: 13 g/m² in the postconditioning group vs. 21 g/m² in the control group; p=0.01)52. Another study with 76 STEMI patients showed that postconditioning did not reduce IS assessed by CMR on day six to nine (median IS as a percentage of the area at risk: 47% in the postconditioning group vs. 44% in the control group, p=NS). In patients with large initial areas at risk, IS was reduced by postconditioning (p<0.001)53. The POstconditioning in ST-Elevation Myocardial Infarction (POSTEMI) trial randomised 272 patients with STEMI within six hours of pain onset to ischaemic postconditioning (four cycles of one-minute reocclusion starting one minute after opening followed by stenting) or control. IS - measured with CMR at four days - did not differ in the postconditioning or control group (median: 14.4% vs. 13.5% of the left ventricle; p=0.18)54. The recent meta-analyses have given conflicting messages with regard to the impact of postconditioning on IS, ventricular function and clinical outcome after PPCI55-57. The impact of ischaemic postconditioning on the clinical outcomes after PPCI is currently under investigation in the DANAMI-3 trial (ClinicalTrials.gov Identifier: NCT01435408).
Remote ischaemic conditioning (repetitive cycles of ischaemia/reperfusion in a tissue remote from the heart) has shown significant cardioprotective effects (reducing IS or improving ST-segment resolution) when performed in patients with STEMI in the ambulance en route to a PPCI centre58, upon hospital arrival prior to PPCI59, or at the time of PPCI60. The recently published LIPSIA CONDITIONING trial randomised 696 patients with STEMI to combined intra-hospital remote ischaemic conditioning plus postconditioning plus PCI or postconditioning plus PCI or PCI alone (three groups). The salvage index assessed by CMR (primary outcome) was significantly greater in the combined conditioning group compared with the control (PCI alone) group (49 [interquartile range: 30-72] vs. 40 [16-68], p=0.02). Postconditioning (plus PCI) failed to improve myocardial salvage when compared with PCI alone. IS or microvascular obstruction or the composite of six-month death, reinfarction or new heart failure showed no difference between the groups61. Despite these results, the clinical benefit of remote conditioning remains unexplored and is currently under investigation.
Conclusions
The use of mechanical strategies to enhance myocardial salvage in the setting of PPCI in patients with STEMI has produced mixed results. Coronary stenting and direct stenting have become almost default approaches of reperfusion during PPCI procedures. Evidence available on the use of mesh-covered, self-expanding stents, deferred stenting or left ventricular assist devices is scant and their use in the setting of PPCI remains limited. Based on existing evidence, the use of mechanical thrombectomy, distal protection devices or routine use of intra-aortic balloon counterpulsation in the setting of PPCI does not seem to result in clinical benefit. Although manual aspiration may improve indices of tissue reperfusion, recent research has shown no clinical benefit of routine use of this strategy in patients with STEMI undergoing PPCI. The use of ischaemic conditioning in the setting of PPCI remains at an investigational stage and needs further research.
| Impact on daily practice Several mechanical strategies have been used to enhance myocardial salvage during primary percutaneous coronary intervention (PCI) in patients with STEMI. Mechanistically they are deemed to optimise acute procedural success, attenuate distal embolisation of thrombotic-atherosclerotic debris, alleviate microvascular obstruction or provide haemodynamic support; all of them are supposed to enhance myocardial salvage during primary PCI procedures. With the exception of coronary (direct) stenting, all other mechanical strategies used either have produced suboptimal clinical results or remain poorly investigated. Although most mechanical strategies are still being investigated for potential clinical utility, their clinical efficacy remains unproven and their use in daily practice of primary PCI remains rather limited or contraindicated. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

