
Abstract
Aims: The inability to optimise stent expansion fully whilst simultaneously preventing distal embolisation during ST-elevation myocardial infarction (STEMI) remains a clinical conundrum. We aimed to describe a newly devised angiographic strategy of “forward” and “back” aspiration that leads to more complete thrombus removal and prevention of distal embolisation, to allow high-pressure post-dilatation of the implanted stent to be performed.
Methods and results: Forward aspiration was conducted with a conventional aspiration thrombectomy catheter, with bail-out aspiration thrombectomy for angiographically persistent thrombus utilising the larger bore 6 Fr (0.056”) guide catheter extension system (GuideLiner®; Vascular Solutions, Inc., Minneapolis, MN, USA). Back aspiration was undertaken with a deeply intubated GuideLiner or guide catheter with a vacuum induced within, extending to the inflated angioplasty balloon, to allow for proximal embolic protection during balloon deflation during all stages of the PCI procedure, including high-pressure post-dilatation of the stent to the visually estimated reference vessel diameter (RVD). Over a six-month period 30 consecutive cases were undertaken during working hours. Bail-out GuideLiner-assisted aspiration thrombectomy was performed in 9/30 cases because of inadequate thrombus removal with a conventional aspiration thrombectomy catheter. Back aspiration was performed in all cases. In 27/30 cases high-pressure post-dilatation of the stent was performed. The mean maximum post-dilatation balloon size and mean proximal reference vessel diameter did not significantly differ (3.60±0.41 mm vs. 3.65±0.45 mm, p=0.68). In all cases, implantation +/- post-dilatation of the stent to the visually estimated RVD was achievable without any deterioration in TIMI blood flow or myocardial blush grade.
Conclusions: The strategy of forward and back aspiration to facilitate stent implantation and high-pressure post-dilatation during STEMI appears to be safe and effective. Randomised controlled trials are required to confirm the safety and efficacy of this newly devised angiographic strategy.
Introduction
The inability to attain full stent expansion during primary PCI for STEMI has been associated with adverse outcomes1-5. As compared to elective PCI, stent underexpansion and incomplete stent apposition are both reported to occur much more frequently during STEMI (in up to 30% of cases) and have been linked to an early and long-term risk of stent thrombosis with first- and newer-generation DES1-5. Notably, stent thrombosis is reported to be the most common cause of early and late reinfarction following primary PCI, and strongly associated with cardiac and all-cause mortality3.
Acquired stent malapposition following primary PCI may be caused by incorrect stent sizing due to thrombus behind the struts hampering stent expansion, with the operator often being unwilling to perform high-pressure post-dilatation for fear of distal embolisation and no reflow, vasoconstriction in the acute phase of STEMI with subsequent vasorelaxation in the few hours/days post procedure, and dissolution of thrombus behind stent struts3,5,6. In addition, distal embolisation of atherothrombotic debris is a major determinant of poor reperfusion, microvascular obstruction (MVO) and infarct size in patients undergoing primary PCI for ST-elevation myocardial infarction (STEMI)7. In an attempt to attenuate the risk of distal embolisation occurring during STEMI, operators may rapidly induce platelet blockade with glycoprotein IIb/IIIa inhibitors – at the expense of an increased bleeding risk, particularly in the elderly – or limit/perform low-pressure post-dilatation of the implanted stent – at the expense of potential stent underexpansion8.
At present, fully optimising stent expansion whilst simultaneously preventing distal embolisation and protecting the distal coronary vasculature remains elusive. Here we present pilot data on 30 consecutive cases of primary PCI for STEMI in which we utilised a newly developed angiographic strategy of “forward” and “back” aspiration that leads to more complete thrombus removal and prevention of distal embolisation, to allow high-pressure post-dilatation of the implanted stent to be performed in order to fully optimise stent implantation during STEMI.
Methods
TREATMENT PROTOCOL
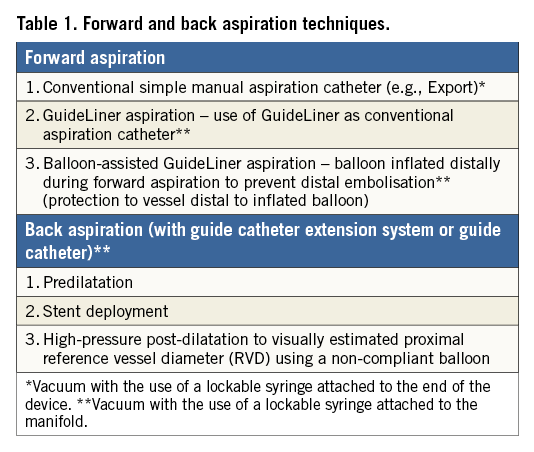
The following treatment protocol was applied in all cases. All procedures were performed transradially using a 6 Fr guiding catheter. Consecutive cases of primary PCI for STEMI undergoing forward and back aspiration (Table 1) were prospectively recruited during working hours. Forward aspiration thrombectomy was undertaken utilising a conventional manual aspiration thrombectomy device (Export® AP Aspiration Catheter; Medtronic, Minneapolis, MN, USA or VMAX Aspiration Catheter; Stron Medical, Winsen, Germany). If angiographically visible thrombus persisted, TIMI thrombus grade 3 (definite thrombus appearing in multiple angiographic views, with greatest dimensions from >1/2 to <2 vessel diameters) or above, bail-out aspiration thrombectomy was performed utilising a guide catheter extension system (GuideLiner; Vascular Solutions, Inc., Minneapolis, MN, USA). Back aspiration was performed with a proximally placed GuideLiner or guide catheter with a vacuum induced within extending to the inflated angioplasty balloon, to allow proximal embolic protection and prevention of distal embolisation during balloon deflation at each stage of the PCI procedure, including high-pressure post-dilatation of the implanted stent to the visually estimated proximal reference vessel diameter (RVD). Intravenous unfractionated heparin was used in all cases (100 U/kg), with a targeted activated clotting time (ACT) of greater than 250 seconds. Glycoprotein IIb/IIIa inhibitors were used at the discretion of the operator. Intracoronary vasodilators were permitted at the discretion of the operator.
RECORDED OUTCOMES
Recorded outcomes included quantitative coronary angiography, TIMI thrombus grade, TIMI blood flow, myocardial blush grades (post stent implantation and post-dilatation) and baseline anatomical and residual SYNTAX scores (www.syntaxscore.com)9,10. All analyses were undertaken by two interventional cardiologists. In cases of disagreement, the opinion of a third interventional cardiologist was sought.
FORWARD ASPIRATION
Following conventional manual aspiration thrombectomy to restore blood flow to the occluded vessel in STEMI, bail-out GuideLiner-assisted manual aspiration thrombectomy was undertaken for TIMI thrombus grade 3 (definite thrombus appearing in multiple angiographic views, with greatest dimensions from >1/2 to <2 vessel diameters) or above.
Two techniques are described.
1) The use of a GuideLiner as a conventional manual aspiration thrombectomy device, with a vacuum induced within using a lockable syringe attached to the manifold (refer to the back aspiration technique detailed below). Care should be taken not to cross the lesion with the GuideLiner device to prevent iatrogenic distal embolisation.
2) Balloon-assisted GuideLiner-assisted manual aspiration thrombectomy utilising an angioplasty balloon inflated distally during forward aspiration thrombectomy to prevent distal embolisation (Figure 1).
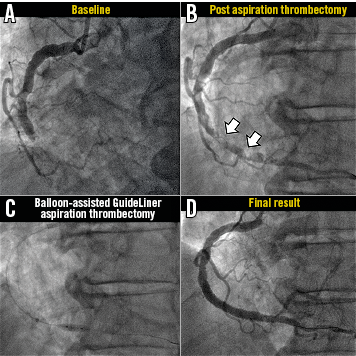
Figure 1. Representative case example of bail-out balloon-assisted GuideLiner-assisted aspiration thrombectomy. A) Baseline angiography demonstrated an acutely occluded mid RCA. B) Following conventional aspiration thrombectomy with an Export device, significant thrombus remained (TIMI thrombus grade 4). C) Balloon-assisted GuideLiner aspiration thrombectomy (with a vacuum induced within the GuideLiner catheter) was undertaken with a balloon inflated distally to protect the distal vasculature. D) Restoration of TIMI 3 flow and normal myocardial blush grades.
With either technique, the GuideLiner is removed with the guide catheter fully intubated in the coronary ostium to prevent any potential embolisation of thrombus to the systemic vasculature. Further blood is then aspirated from the guide catheter to ensure no thrombus remains within the guide catheter.
BACK ASPIRATION
The “back aspiration” technique during STEMI (Figure 2, Figure 3) is performed with predilatation (to restore vessel flow or if further predilatation is required [as per operator judgement]), stent implantation and mandated high-pressure (16 atm) post-dilatation of the implanted stent to the reference vessel diameter (visual estimation). Back aspiration may be performed with either the GuideLiner or the actual guide catheter itself for very proximal lesions.
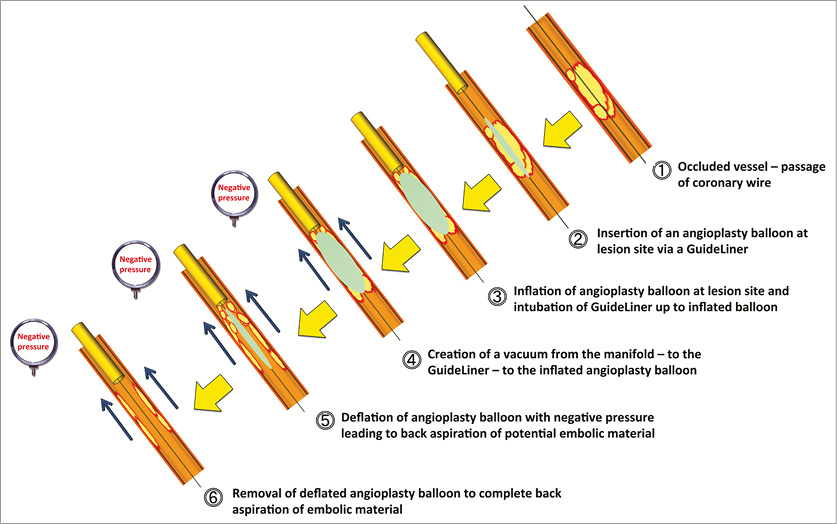
Figure 2. Principle of back aspiration performed during primary PCI. Principle of back aspiration utilising a proximally placed GuideLiner with a vacuum induced within the GuideLiner extending to the inflated angioplasty balloon, to allow proximal embolic protection and prevention of distal embolisation during all stages of the PCI procedure (predilatation to restore vessel blood flow as illustrated [or use of a conventional manual aspiration thrombectomy device] ±further predilatation if clinically indicated), stent implantation and mandated high-pressure (16 atm) post-dilatation to the visually estimated proximal RVD.
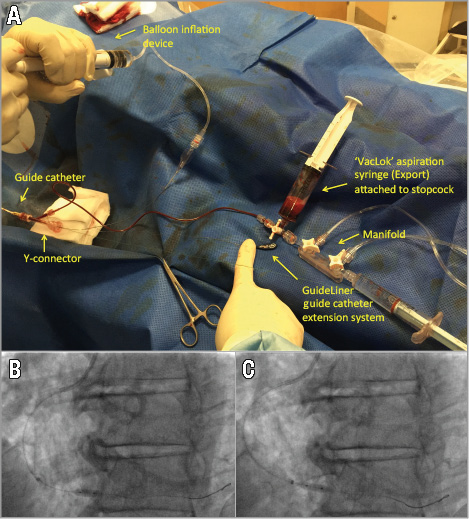
Figure 3. “Back aspiration” technique performed during primary PCI. Note the lockable aspiration syringe (VacLok®; Merit Medical Systems, Inc., South Jordan, UT, USA, available with the manual aspiration thrombectomy catheter) attached to the manifold on full negative pressure with the three-way tap initially closed to the VacLok to allow the creation of a vacuum within the VacLok. Opening the three-way tap from the GuideLiner to the VacLok allows the creation of a vacuum within the GuideLiner catheter (A) extending to the inflated angioplasty balloon (B & C). During back aspiration, the angioplasty balloon/stent is positioned at the landing zone and inflated (B). The GuideLiner catheter is then advanced just proximal to the inflated balloon (C) and a vacuum induced within the GuideLiner and extended up to the inflated balloon by opening the three-way tap from the GuideLiner to the VacLok as described earlier (A). Deflation of the angioplasty balloon, followed by removal of the balloon, allows any potential material to be back aspirated rather than to embolise distally.
1. The GuideLiner is inserted into the guide catheter through a Y-adaptor with haemostasis valve (Tuohy-Borst type) and positioned at the guide catheter tip.
2. A lockable aspiration syringe (available with the simple aspiration thrombectomy catheter) is attached to the stopcock and all connections on the manifold, stopcock and tubing are checked to safeguard against any loose connections which may compromise the vacuum within the GuideLiner (Figure 3A).
3. The angioplasty balloon/stent is passed through the GuideLiner and positioned in the proximal vessel before the landing zone. The GuideLiner is carefully inserted into the coronary vessel using the shaft of the angioplasty balloon/stent as support to facilitate the GuideLiner insertion into the coronary vessel. The balloon/stent is positioned at the landing zone and inflated (Figure 3B). The GuideLiner catheter is then advanced just proximal to the inflated balloon (Figure 3C). Performing this latter manoeuvre will ensure the GuideLiner catheter will bypass any proximal side branches and aspirate blood from the landing zone of the stent when the balloon is deflated, and will therefore fully maximise aspiration flow rates. N.B. gentle retraction of the inflated angioplasty balloon can facilitate insertion of the GuideLiner catheter into the coronary vessel; this is particularly useful for insertion through the more proximal segment of the vessel, particularly if tortuous.
4. Inflation* of the angioplasty balloon is followed by the withdrawal and locking of the plunger of the lockable aspiration syringe (attached to the stopcock) to full negative pressure in order to create a vacuum within the GuideLiner. This is immediately followed by opening of the stopcock to extend the vacuum from the GuideLiner to the inflated angioplasty balloon.
5. Full deflation* of the angioplasty balloon is followed by immediate removal of the angioplasty balloon into the guide catheter under back aspiration protection from the GuideLiner (Figure 2). During this process gentle forward traction is placed on the GuideLiner catheter to assist in preventing inadvertent withdrawal of the GuideLiner during balloon removal. This entire process allows the vacuum (induced within the GuideLiner extending to the angioplasty balloon) to arrest forward flow of blood in the coronary vessel, and, importantly, to aspirate any material that would otherwise have been embolised distally. Notably, as soon as the angioplasty balloon is removed from the GuideLiner (following full deflation), aspiration flow rates will be maximised since removal of the shaft of the angioplasty balloon from the GuideLiner will allow utilisation of the entire lumen of the GuideLiner (internal bore diameter: 6 Fr 0.056” [1.42 mm], 7 Fr 0.062” [1.57 mm]) to aspirate blood, based on Poiseuille’s law (of all the factors that affect blood flow through a catheter, the radius is the most potent since the flow rate is proportional to its 4th power).
6. The lockable aspiration syringe attached to the stopcock is allowed to fill with blood to ensure any potential coronary aspirate within the system is removed.
7. Following completion of the procedure under back aspiration protection for each step (predilatation to restore vessel blood flow±further predilatation if clinically indicated, stent implantation, and mandated high-pressure post-dilatation [≥16 atm] to the visually estimated proximal RVD), the GuideLiner is removed with the guide catheter fully intubated in the coronary ostium (inserted over the GuideLiner to minimise any trauma to the vessel ostium) to prevent any potential embolisation of thrombus to the systemic vasculature. Further blood is aspirated from the guide catheter to ensure no thrombus remains within the guide catheter.
*The contrast/saline mix used to inflate the angioplasty/stent balloons should be in a 25 (contrast) to 75 (saline) ratio. This allows more rapid balloon deflation and removal, thus maximising back aspiration flow rates and potential prevention of distal embolisation.
Results
Over a six-month period (June to December 2014), 30 consecutive cases of GuideLiner-assisted primary PCI for STEMI were undertaken by two operators (Table 2). Twenty-four out of 30 (80%) cases were male, 6/30 (20%) cases were female, the mean age was 64.4±15.2. The mean baseline SYNTAX score was 13.6±7.1, and the residual SYNTAX score following revascularisation was 5.6±8.0. TIMI flow was 0 at the start of the procedure in 17/30 cases (57%). Two out of 30 cases (7%) were performed in cardiogenic shock and 1/30 cases (3%) was performed for very late stent thrombosis. Glycoprotein IIb/IIIa inhibitors were used in 25/30 (83%) cases.
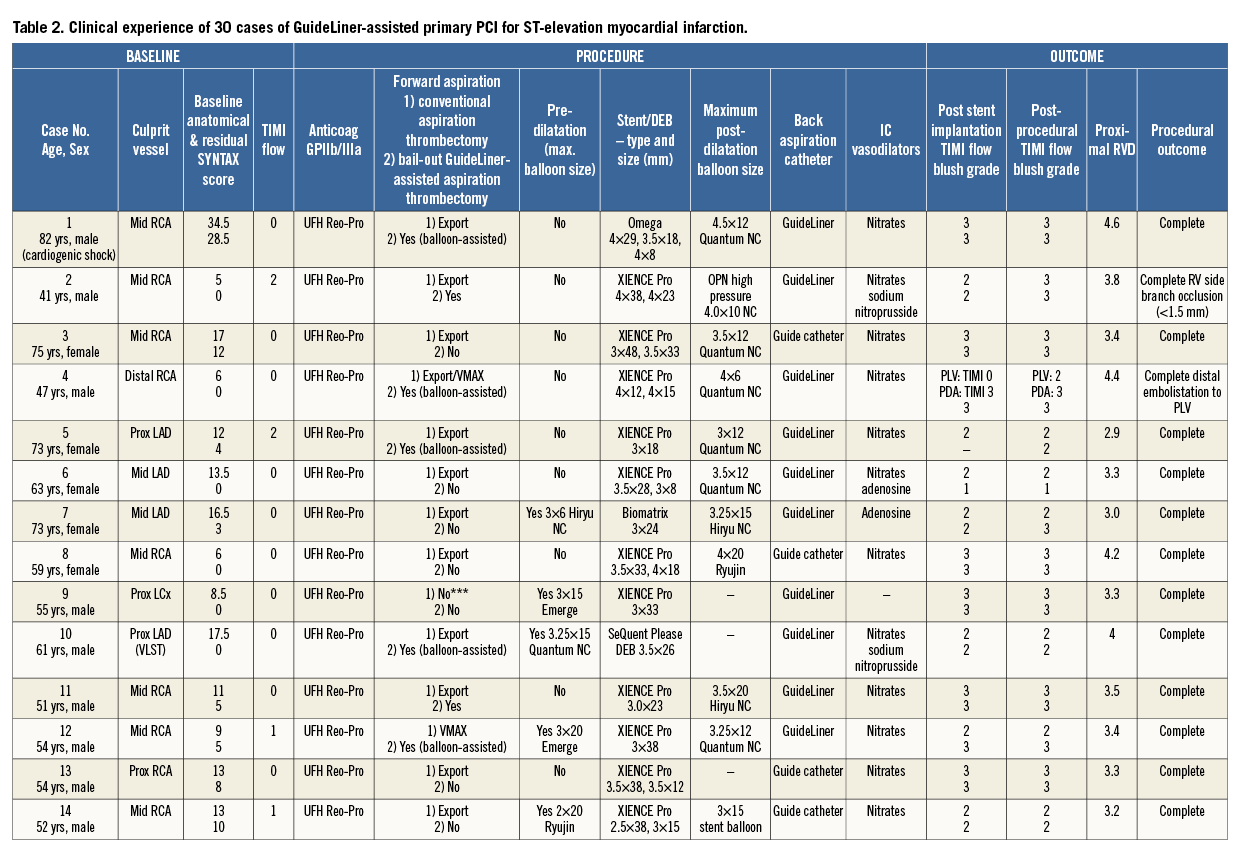
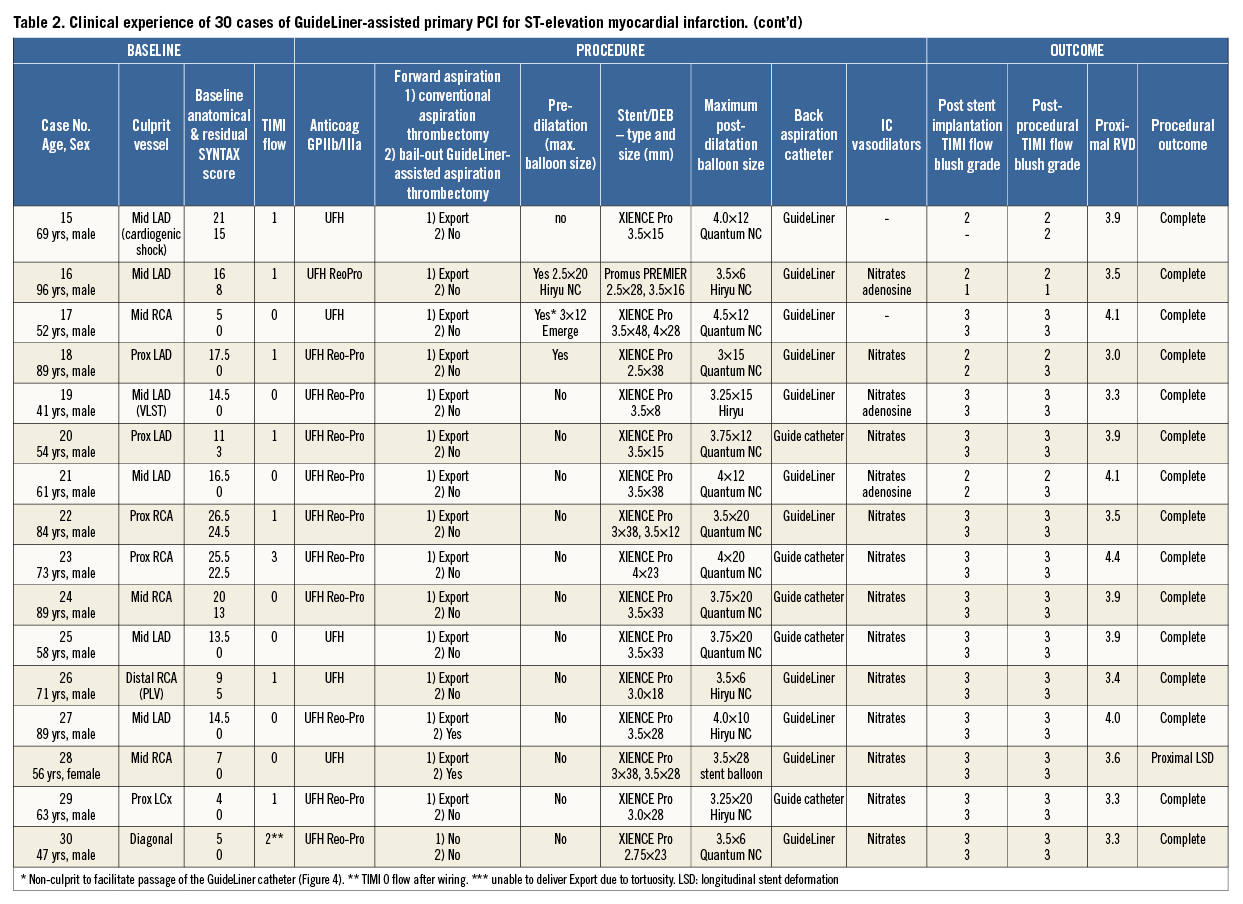
FORWARD ASPIRATION
Bail-out GuideLiner-assisted aspiration thrombectomy was performed in 9/30 cases (30%) for inadequate thrombus removal with a conventional aspiration thrombectomy device (Table 2). Four out of nine cases underwent bail-out GuideLiner-assisted aspiration thrombectomy without balloon assistance. Five out of nine cases underwent bail-out GuideLiner-assisted aspiration thrombectomy with balloon assistance to protect the distal vasculature. In one case of balloon-assisted GuideLiner aspiration thrombectomy (Table 2, Case 4), in which the distal balloon was inflated in the posterior descending artery (PDA) of an RCA, distal embolisation occurred in the posterior left ventricular branch (PLV), warranting further conventional aspiration thrombectomy (Export Aspiration Catheter) to the PLV to restore flow.
BACK ASPIRATION
Back aspiration was performed in all cases. In 21/30 cases (70%) back aspiration was performed via a GuideLiner catheter and in 9/30 cases (30%) via a deeply intubated guide catheter. The guide catheter was predominantly used for back aspiration for RCA lesions (6/9 cases), whereas the GuideLiner was used in all three coronary vessels. Representative case examples are shown in Figure 4 and Figure 5.
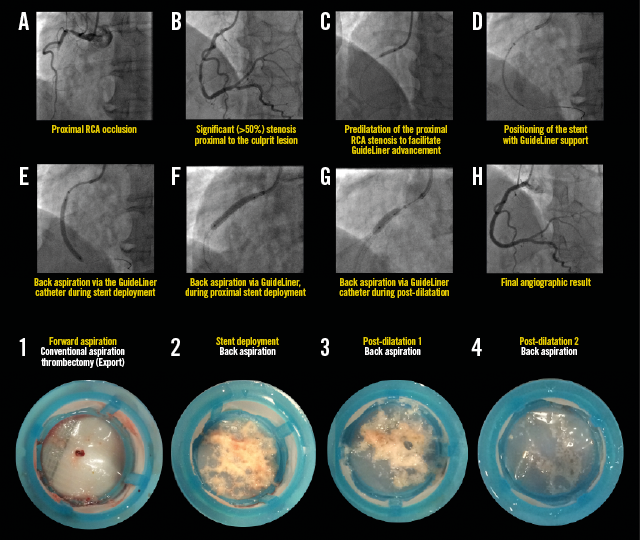
Figure 4. Representative example illustrating all steps in the primary PCI procedure for STEMI, demonstrating the back aspiration technique (A-H). The lower images illustrate coronary aspirates taken during forward (1st image) and back aspirations (2nd to 4th images). Note the decreasing volume of back aspirate following stenting (2nd image) and successive post-dilatations (3rd and 4th images) (For details of procedure refer to Table 2, Case 17).
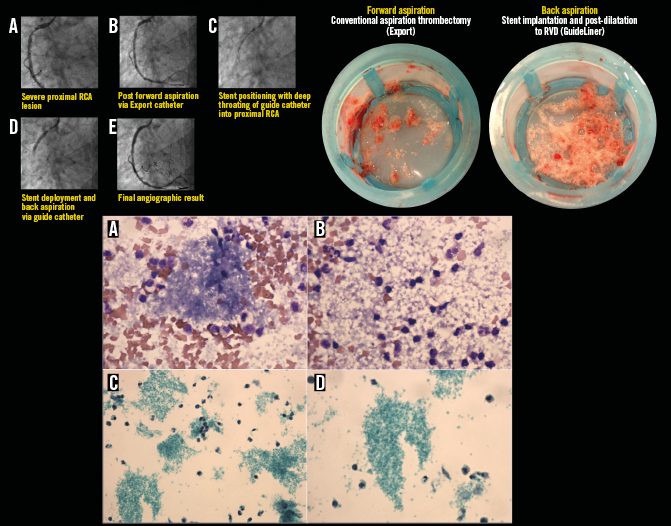
Figure 5. Representative example illustrating all steps in the primary PCI procedure for STEMI, demonstrating the back aspiration technique using the right coronary artery (RCA) guide catheter. A) At the start of the procedure a severe proximal RCA lesion was evident with TIMI 3 flow. B) Forward aspiration was undertaken with an Export catheter. C) & D) Back aspiration was undertaken with deep throating of the guide catheter, followed by post-dilatation (not illustrated). E) TIMI 3 flow at the end of the procedure. Corresponding coronary aspirates (upper right image) following forward (left) and back (right) aspiration are shown, confirming successful thrombus aspiration during forward and back aspiration. Lower images represent corresponding cytology for back aspiration. Cytospin preparations using May-Grünwald-Giemsa stain (×600 magnification, [a & b], and SurePath preparations using Papanicolaou stain (×400 [c] and ×600 [d] magnification) are shown. Granular material consistent with abundant platelet clumps are predominantly seen interspersed with red blood and inflammatory (lymphocytes and neutrophils) cells. (For details of procedure refer to Table 2, Case 23).
POST-DILATATION
In 27/30 cases (90%) high-pressure post-dilatation of the stent was performed to the visually estimated proximal RVD; in the remaining 3/30 cases (10%) the operator’s judgement was that the deployed stent matched the proximal RVD. The mean maximum post-dilatation balloon size and mean proximal reference vessel diameter as assessed by QCA did not significantly differ (3.60±0.41 mm vs. 3.65±0.45 mm, p=0.68).
ANGIOGRAPHIC OUTCOMES
In all cases, implantation±post-dilatation of the stent to the visually estimated RVD was achievable without any deterioration in TIMI blood flow or myocardial blush grade from that seen after stent implantation. In 27/30 cases (90%) angiographic outcomes were successful. In the remaining 3/30 cases (10%): one case resulted in right ventricular branch (<1.5 mm diameter) occlusion with no clinical sequalae (Table 2, Case 2); in the second case there was distal embolisation to the PLV branch following bail-out balloon-assisted GuideLiner aspiration thrombectomy due to the balloon being inflated in the PDA and thereby only protecting this vessel (Table 2, Case 4); and the third case resulted in proximal longitudinal stent deformation by the GuideLiner catheter following inadvertent deep throating of the GuideLiner catheter following stent (3.5 mm XIENCE PRO; Abbott Vascular, Santa Clara, CA, USA) implantation. Further post-dilatation of the stent was undertaken to correct this abnormality (Table 2, Case 28).
A representative selection of case examples from Table 2 is shown in Appendix Figure 1-Appendix Figure 7.
Discussion
The main findings of this feasibility study are as follows. First, forward aspiration using the guide catheter extension system (with or without balloon assistance) during STEMI provides an additional bail-out strategy for angiographically persistent thrombus compared to conventional manual aspiration thrombectomy catheters. Second, the back aspiration strategy using the guide catheter/GuideLiner allowed proximal embolic protection during all stages of the primary PCI procedure, and permitted routine high-pressure stent post-dilatation to the visually estimated RVD without any angiographically detectable impairment in TIMI flow or myocardial blush grade. Third, the forward and back aspiration strategy appeared safe with a low complication rate.
Based on the macro/microscopic appearance of the forward and back aspirates, we hypothesise that forward aspiration would allow removal of red thrombus and potential macroembolisation, whilst back aspiration would prevent microembolisation and the potential for no-reflow. This may be a possible explanation for the negative results of the two large multicentre randomised trials, TOTAL11 and TASTE12, which failed to associate routine manual aspiration thrombectomy in STEMI (compared to a bail-out strategy) with improved clinical outcomes. In addition, although the angiographic substudy of TOTAL13 demonstrated that distal embolisation (defined as “pruning” of the small distal vessels) occurred more frequently with balloon dilatation (compared to routine aspiration thrombectomy), this did not translate into any clinical benefit, presumably because any significant macroembolisation underwent bail-out aspiration thrombectomy. Furthermore, myocardial blush grades did not significantly differ between the thrombectomy and balloon angioplasty arms13, supporting the concept that aspiration thrombectomy itself does not protect against microembolisation and is only suitable as a bail-out strategy to tackle macroembolisation of sizeable vessels.
Safety
The potential safety concerns of deeply intubating a guide catheter or guide catheter extension system should be borne in mind when performing forward or back aspiration. Very proximal coronary artery disease (particularly in the RCA) allowed the use of a guide catheter for back aspiration, whereas more distal coronary artery disease required the use of a guide catheter extension system. The softer, atraumatic tip of the GuideLiner guide catheter extension allowed deeper insertion into coronary vessels without any safety concerns with respect to coronary artery dissections. These findings are corroborated by registries reporting an increase in the procedural success rates of complex PCI through very deep intubation into the coronary vessel with a very low complication rate14-17. Following stent implantation, care should be taken not to allow the GuideLiner catheter inadvertently to move forward to prevent longitudinal stent compression18, as occurred in one case during the present study. In addition, we cannot exclude the possibility that invasive assessment of the coronary microvasculature through coronary physiology measurements19 may provide a better insight into the effects of distal embolisation compared to the angiographic strategies employed in the present study.
Limitations
The present study presents non-randomised pilot data from a single-centre registry. Although the pilot data demonstrate the potential safety and efficacy of the forward and back aspiration approaches, randomised trials using core laboratories to analyse the data are required to confirm the safety and efficacy of this newly described angiographic strategy. It was not possible to quantify the amount of thrombus retrieved.
Conclusion
The strategy of forward and back aspiration during primary PCI for STEMI to facilitate implantation and post-dilation of the stent to the visually estimated RVD appears to be safe and effective. Randomised controlled trials are required to assess the safety and efficacy of this newly devised angiographic strategy.
| Impact on daily practice In daily practice during primary PCI for ST-elevation myocardial infarction (STEMI), fully optimising stent expansion, whilst simultaneously preventing distal embolisation and protecting the distal coronary vasculature, remains a clinical problem. Notably, both stent underexpansion and distal embolisation are major determinants of adverse short- and longer-term outcomes, including mortality; routine manual aspiration thrombectomy has however recently been shown to be ineffective. Here we present a newly developed angiographic strategy of “forward” and “back” aspiration that leads to more complete thrombus removal and prevention of distal embolisation, to allow high-pressure post-dilatation of the implanted stent to be performed, with the potential for significant patient benefit. Randomised controlled trials are required to confirm the efficacy and safety of this newly developed angiographic strategy. |
Guest Editor
This paper was guest edited by Manel Sabaté, MD, PhD, Cardiology Department, Hospital Clínic de Barcelona, Barcelona, Spain.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
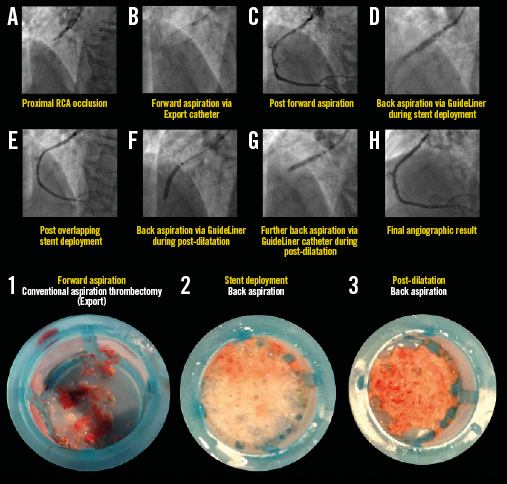
Appendix Figure 1. Representative example illustrating all steps in the primary PCI procedure for STEMI with proximal RCA occlusion. The back aspiration technique using the GuideLiner catheter is shown in panels A) to H). The lower images illustrate coronary aspirates taken during forward (image 1) and back aspirations during stent deployment (image 2) and post-dilatation (image 3). For details of the procedure refer to Table 2, Case 22.
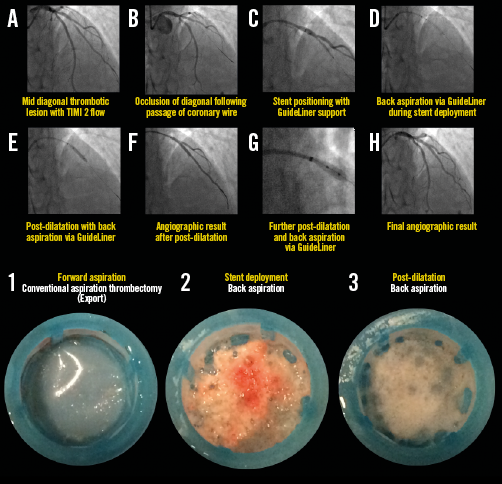
Appendix Figure 2. Representative example illustrating all steps in the primary PCI procedure for STEMI with diagonal occlusion (TIMI 2 flow). Forward and back aspiration (with the GuideLiner catheter) techniques are illustrated in panels A) to H), with corresponding coronary aspirates (lower images). Note that forward aspiration yielded no thrombotic debris, whereas there was significant back aspirate during stent deployment and post-dilatation. For details of the procedure refer to Table 2, Case 30.
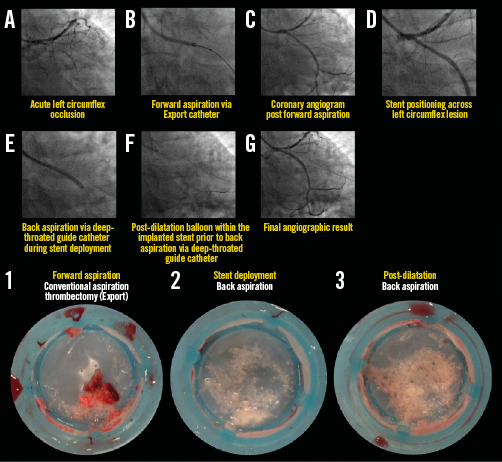
Appendix Figure 3. Representative example illustrating all steps in the primary PCI procedure for STEMI with proximal left circumflex occlusion. The back aspiration technique using the guide catheter as the back aspiration catheter (A-G). The lower images illustrate coronary aspirates taken during forward (image 1) and back aspirations for stent deployment (image 2) and post-dilatation (image 3). For details of the procedure refer to Table 2, Case 29.
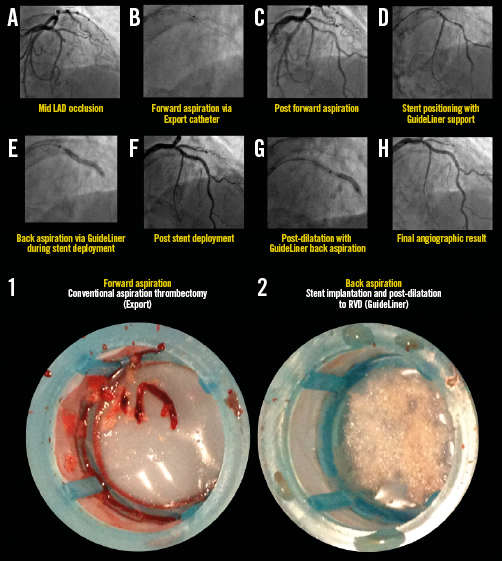
Appendix Figure 4. Representative example illustrating all steps in the primary PCI procedure for STEMI with mid LAD occlusion. Forward and back aspiration techniques, using the GuideLiner catheter, are illustrated in panels A) to H). The corresponding coronary aspirates are shown in the lower images. For details of the procedure refer to Table 2, Case 6.
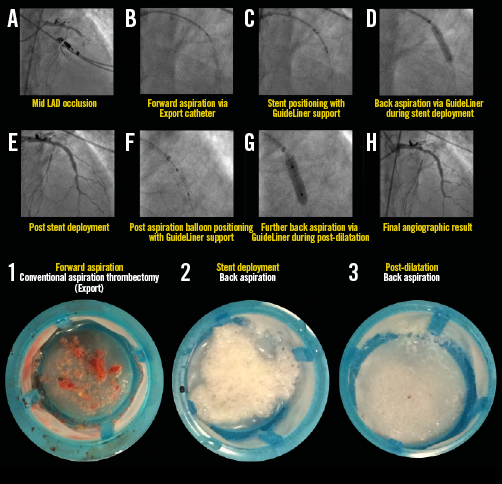
Appendix Figure 5. Representative example illustrating all steps in the primary PCI procedure for STEMI with mid LAD occlusion. The back aspiration technique and positioning of the GuideLiner catheter are shown in panels A) to H). The lower images illustrate coronary aspirates taken during forward (image 1) and back aspirations during stent deployment (image 2) and post-dilatation (image 3). For details of the procedure refer to Table 2, Case 15.
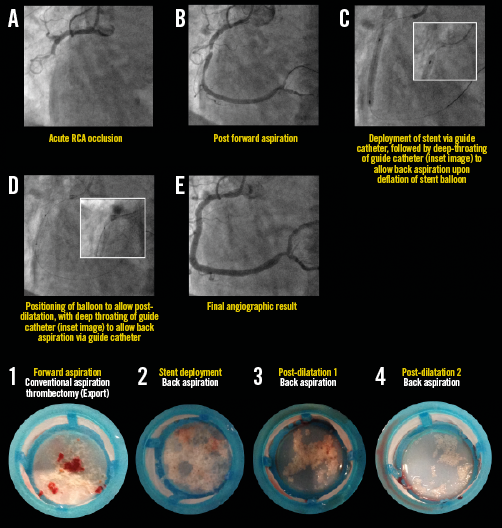
Appendix Figure 6. Representative example illustrating all steps in the primary PCI procedure for STEMI with mid RCA occlusion. The guide catheter is used here as the back aspiration catheter (A-E). The lower images illustrate coronary aspirates. For details of the procedure refer to Table 2, Case 2.
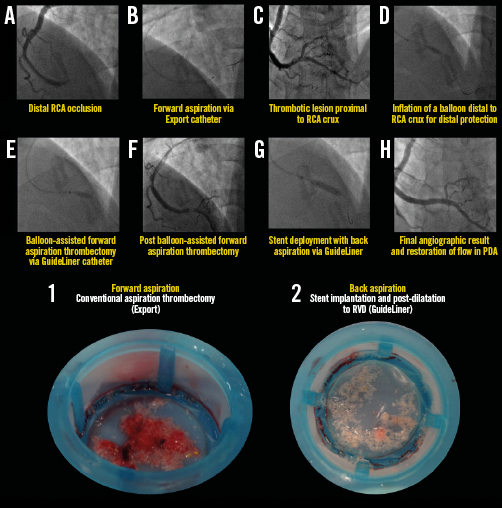
Appendix Figure 7. Representative example illustrating all steps in the primary PCI procedure for STEMI with distal RCA occlusion. Forward and back aspiration (with the GuideLiner catheter) techniques are illustrated in panels A) to H). Corresponding coronary aspirates are shown in the lower images. For details of the procedure refer to Table 2, Case 4.

