Abstract
Aims: Data suggest that there is a variable use of thrombectomy during primary percutaneous coronary interventions (PPCI). We sought to evaluate practices during PPCI for ST-elevation myocardial infarction (STEMI), including the use of aspiration thrombectomy, and to determine the feasibility of conducting a definitive aspiration thrombectomy trial.
Methods and results: A 27-item online survey was distributed to 1,607 interventional cardiologists internationally. A total of 461 responses were received. During PPCI, aspiration thrombectomy is used routinely by 36% of respondents, and selectively by 60%. Twenty-five percent of respondents reported experiencing a complication related to thrombectomy including: vessel dissection (13%), bringing thrombus back into left main coronary artery from target vessel (5%), stroke or transient ischaemic attack (2%), and coronary artery perforation (1%). The vast majority of respondents (89%) believe that a confirmatory aspiration thrombectomy trial is needed and 85% would be willing to randomise patients in such a trial.
Conclusions: The majority of interventional cardiologists surveyed are not using thrombectomy routinely during PPCI. The survey results suggest that a large, confirmatory thrombectomy trial is needed and feasible in the current era. The survey also highlights a significant level of variability and underutilisation of other evidence-based therapies during PPCI.
Abbreviations
GP: glycoprotein
IQR: interquartile range
MRA: multiple responses accepted
PPCI: primary percutaneous coronary intervention
TIA: transient ischaemic attack
TIMI: thrombolysis in myocardial infarction
STEMI: ST-elevation myocardial infarction
Introduction
New therapies may not be adopted widely in clinical practice due to a variety of factors, including: the perception that current evidence is insufficient to support a change in practice, the cost of new therapies, system or institutional barriers, and a lack of familiarity and training with new agents or procedures.
Thrombectomy during primary percutaneous coronary interventions (PPCI) for ST-elevation myocardial infarction (STEMI) has a class IIa recommendation in American and European STEMI guidelines1,2. The recommendation for thrombectomy use is not stronger because the evidence is derived predominantly from a single-centre trial, the TAPAS trial, which was powered for a surrogate outcome but showed an unexpected large mortality reduction associated with thrombectomy3. Data from the American College of Cardiology National Database Registry suggest that thrombectomy was used in only 19% of PPCI cases for STEMI between 2009 and 20104. It is important to determine whether this finding is due in part to a perception amongst clinicians that further evidence is needed to support a change in practice.
We undertook an international survey to assess: perceptions regarding the current level of evidence for thrombectomy during
PPCI, prevalence of thrombectomy use and practice patterns, variations in the use of direct stenting and periprocedural pharmacology, and choice of vascular access during PPCI for STEMI.
Methods
We designed a 27-item closed survey to assess PPCI practices as well as the current extent and patterns of use of aspiration thrombectomy internationally. The questions addressed: 1) prevalence of aspiration thrombectomy use, 2) preferences for thrombectomy devices and adjunctive therapies, 3) complications of aspiration thrombectomy, 4) criteria for aspiration thrombectomy use, and 5) perceived need for an aspiration thrombectomy trial and willingness to randomise patients to such a trial. The survey also assessed the use of other PPCI strategies including: 1) glycoprotein (GP) IIb/IIIa inhibitors and bivalirudin, 2) pharmacologic agents to prevent no-reflow, 3) direct stenting, and 4) vascular access site preference (radial versus femoral access).
The project was approved by the Hamilton Health Sciences Research Ethics Board. We used Internet-based software (Survey Monkey, Palo Alto, CA, USA) to design and distribute the survey. During the development phase, the survey was sent to 14 experienced cardiologists for feedback on its readability, comprehensiveness, time for completion, and validity and relevance of questions. The original version was then modified and recirculated for further feedback resulting in no additional changes.
The survey was distributed in English via e-mail to practising interventional cardiologists who were part of the international Population Health Research Institute investigator network (n=815, including investigators from Argentina, Brazil, Canada, Czech Republic, France, Germany, Greece, Italy, India, Netherlands, Russia, South Korea, Spain, United Kingdom, and the United States), DUKE STEMI investigators within the United States (n=150), members of the Canadian Association of Interventional Cardiology (n=130), and Polish (n=485), Czech (n=65), and Nordic (n=200) interventional cardiology working groups. There were 238 individuals who were on more than one e-mail list and these individuals were only counted once, for a total of 1,607 cardiologists surveyed. The survey was first sent to participants on February 1st, 2010, and a follow-up request was sent two weeks later. Survey responses were collected from February 1st to November 30th, 2010.
Statistical analysis
The results are presented as medians and interquartile ranges for continuous variables and percentage of total responses for categorical variables. The results were divided into three regions: North America, Europe, and other. Trends across regions were analysed using the χ2 test and Kruskal-Wallis test for categorical and continuous variables, respectively, using JMP (version 9; SAS Institute Inc., Cary, NC, USA).
Results
A total of 461 responses were received from 1,607 cardiologists contacted, consistent with a response rate of 29%.
DEMOGRAPHICS OF RESPONDENTS
Respondents were predominantly from Europe (66%), North America (24%), South America (5%), and India (2.3%). The demographics of respondents are summarised in Table 1. Sixty percent of respondents work in university-based academic centres, while the remaining 40% practise in non-university-based tertiary centres or private institutions. The majority of respondents were high-volume operators with 66% performing more than 200 PCI procedures per year. The respondents were also high-volume STEMI operators with a median of 50 PPCIs per year, and worked in centres performing a median of 250 PPCIs for STEMI per year. Most respondents (86%) had at least five years of experience performing PCI for STEMI, with 54% having more than 10 years of experience.
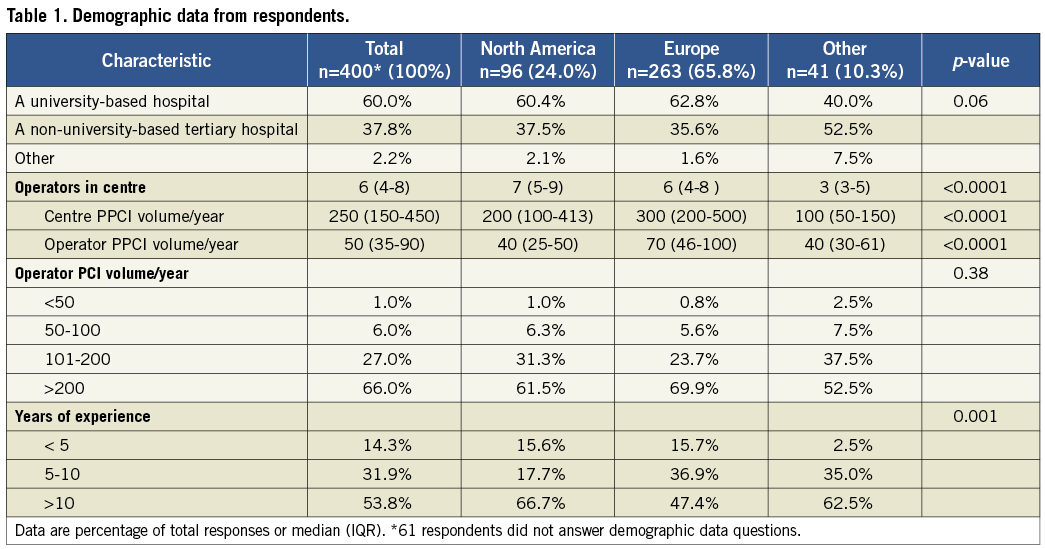
Primary PCI strategies
USE OF ASPIRATION THROMBECTOMY
The reported median use of aspiration thrombectomy during the 12 months preceding the survey was 60% of all PPCI for STEMI (Table 2). Only 36% of respondents reported using aspiration thrombectomy routinely while an additional 60% used this procedure selectively. Four percent of respondents reported rare or no use of aspiration thrombectomy. The median use of aspiration thrombectomy was 80%, 30%, and 10% of all PPCI for STEMI in those reporting routine, selective, and rare use of thrombectomy, respectively. Reasons for not using thrombectomy included a perceived lack of evidence supporting a clear benefit, as well as increased procedural time and costs. There was no significant difference in the prevalence of aspiration thrombectomy use among regions (Table 2). Procedural costs affect the aspiration thrombectomy practice of 14% of respondents, with cost being a particularly important factor in centres outside North America and Europe (Table 2). Respondents who indicated an influence of costs on practice identified the following financial obstacles: lack of coverage for thrombectomy devices by government insurance plans, lack of patient private health insurance, and limited procedural budgets.
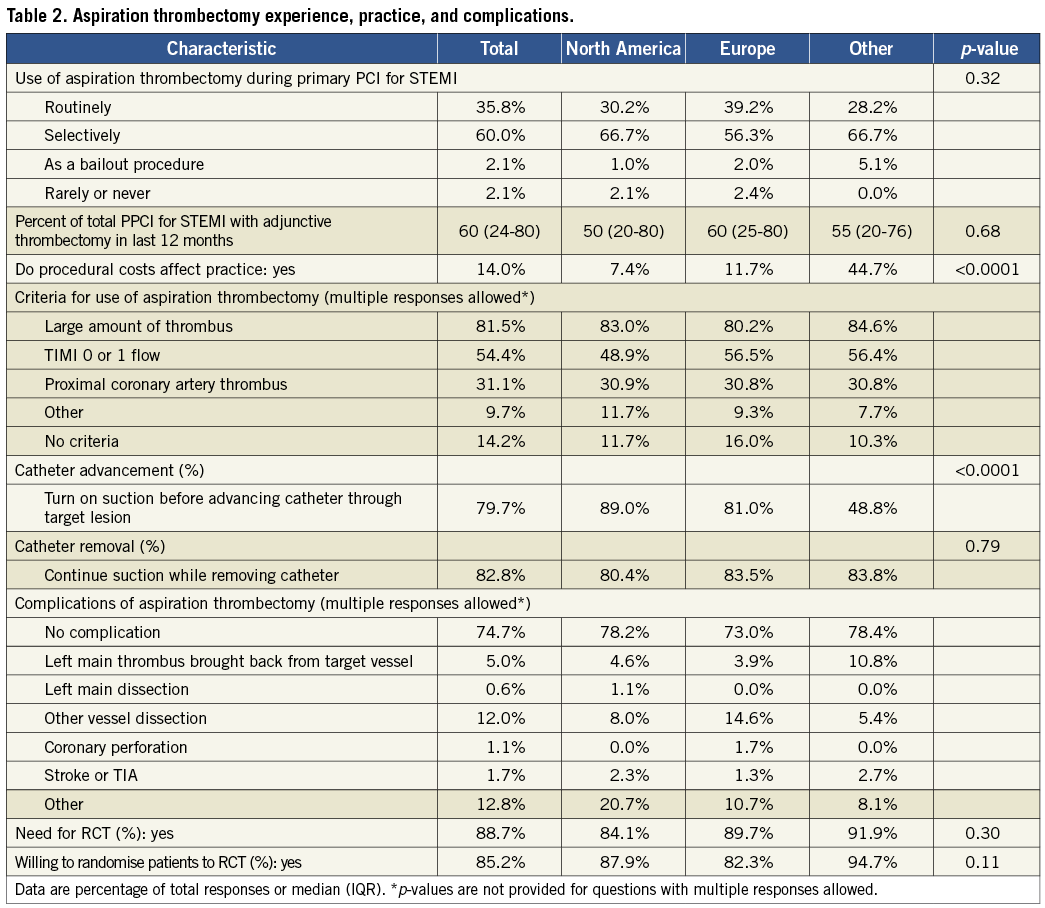
CRITERIA FOR ASPIRATION THROMBECTOMY USE
Eighty-six percent of respondents stated that they have specific criteria for the use of aspiration thrombectomy (Table 2). Common criteria for thrombectomy use include: a large amount of thrombus (82%), TIMI 0 or 1 flow (54%), and proximal location of the thrombus in the coronary artery (31%). Additional criteria included large infarct-related artery, no-reflow, and contraindications to GP IIb/IIIa inhibitors.
ASPIRATION THROMBECTOMY PROCEDURAL TECHNIQUES, DEVICES AND COMPLICATIONS
With regard to crossing the lesion, 80% of respondents initiate suction before advancing the catheter through the target lesion with significant differences among regions (Table 2). Twenty percent initiate suction after advancing the catheter through the target lesion and aspirate while pulling back the catheter. When removing the device, 17% of operators turn off suction before removing the catheter from the target vessel while 83% maintain suction while doing so.
The Export catheter (6 Fr) (Medtronic CardioVascular, Santa Rosa, CA, USA) was the most commonly used thrombectomy device, utilised by 77% of operators with 31% using it exclusively. Other widely used devices included: 1) Diver CE (Invatec S.p.A., Roncadelle, Italy) used by 26%, with 5% using it exclusively, 2) Pronto V3 catheter (Vascular Solutions, Minneapolis, MN, USA) used by 25% of respondents, with 6% using it exclusively, and 3) Export XT 7 Fr (Medtronic CardioVascular, Santa Rosa, CA, USA) used by 15% of respondents, with no respondents using it exclusively.
Twenty-five percent of respondents reported experiencing at least one serious complication related to the use of aspiration thrombectomy including: thrombus aspirated back from target vessel into left main coronary artery (5%), stroke or transient ischaemic attack (TIA) (2%), left main coronary artery dissection (0.6%), and vessel dissection other than left main (12%). Additional complications identified included distal embolisation, air embolism, and no-reflow.
NEED FOR A LARGE-SCALE RANDOMISED TRIAL FOR ASPIRATION THROMBECTOMY
The majority (89%) of respondents stated that a large, definitive trial of aspiration thrombectomy is needed while only 11% stated that the existing evidence supports the use of thrombectomy as the standard of care. Eighty-five percent of respondents would be willing to randomise patients into a large, definitive trial of aspiration thrombectomy during PPCI.
ADJUNCTIVE PHARMACOLOGICAL THERAPY DURING PPCI
GP IIb/IIIa inhibitors are used routinely by 36% of respondents, selectively by 53%, and seldom or never by 11%. There were significant regional variations in the use of GP IIb/IIIa inhibitors (Table 3, p=0.02) with a higher rate of routine use in North America (49%) compared to Europe (31%) and other regions (33%).
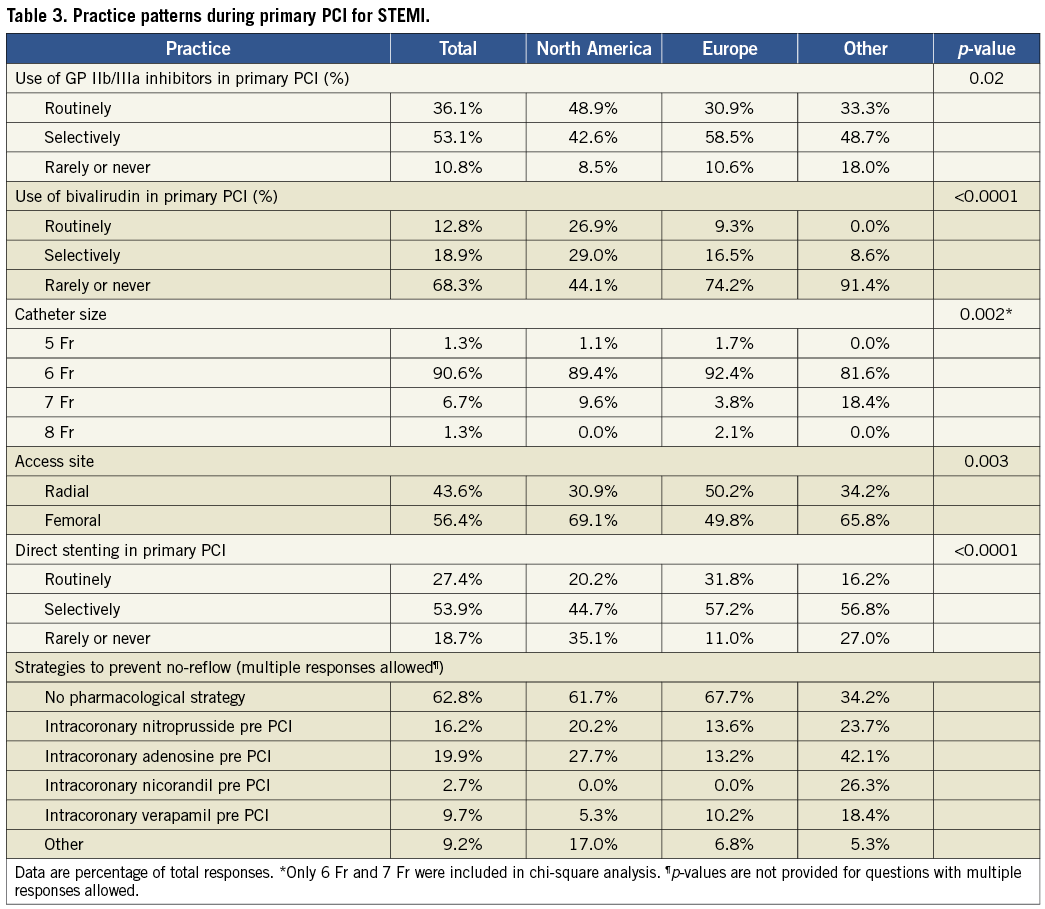
Bivalirudin is used routinely and selectively by 13% and 19% of operators, respectively, and seldom or never by 68%. Routine use of bivalirudin is more common in North America (27%) than in Europe (9%) and other regions (0%) (p<0.0001). Thirty-seven percent of respondents reported pre-treating with microvascular vasodilators to prevent no-reflow during PPCI. In order of prevalence, pre-treatment with prophylactic intracoronary adenosine, nitroprusside, verapamil, and nicorandil are used by 20%, 16%, 10%, and 3% of operators, respectively.
RADIAL VS. FEMORAL ACCESS AND SHEATH SIZE
During PPCI, 56% of operators prefer femoral access, while the remaining 44% prefer a radial route. There was a statistically significant difference in access route preference among regions (Table 3, p=0.003) with a stronger preference for radial access in Europe (50%) compared to North America (31%) and other regions (34%). The majority of operators (91%) prefer using 6 Fr catheters, while 6.7%, 1.3%, and 1.3% prefer 7 Fr, 8 Fr, and 5 Fr catheters, respectively.
DIRECT STENTING DURING PPCI
Direct stenting during PPCI is performed routinely by 27% of operators and selectively by 54%. There was a statistically significant difference in the use of this technique among regions (Table 3, p<0.0001), with a higher prevalence of direct stenting in Europe (32%) compared to North America (20%) and other regions (16%). One fifth of respondents (19%) seldom or never use direct stenting.
Discussion
This international survey of interventional cardiologists demonstrates that, despite the benefits observed in the TAPAS trial, the majority of interventional cardiologists in contemporary practice are not using aspiration thrombectomy routinely during PPCI. Our survey suggests that complications related to this technique do occur, with approximately one quarter of respondents having experienced a complication from its use. In addition, 89% of respondents believe that a definitive randomised trial is needed, and 85% would randomise patients to such a trial, supporting its feasibility. Finally, there are variations of practice in the use of other strategies during PPCI, including bivalirudin, GP IIb/IIIa inhibitors, direct stenting, and radial access.
The finding that only 36% of operators use thrombectomy routinely is surprising given the large benefit observed in the TAPAS trial and the class IIa recommendation in both American and European guidelines1-3. Data from the American College of Cardiology National Database Registry report thrombectomy use rates of only 19% during PPCI for STEMI, which is much lower than reported by respondents in our survey4. The difference may be the result of selection bias, as we surveyed operators from centres participating in clinical trials, who may be more likely to adopt new practices.
The reasons behind the lower than expected thrombectomy use rates are probably multifactorial. Nearly 90% of operators feel that a large randomised trial is needed in order to validate the routine use strategy. In addition, approximately one quarter of respondents have experienced a serious complication related to thrombectomy use including pulling thrombus back into the left main coronary artery. While these complications may be very uncommon, they could discourage a clinician from utilising the device.
Device manufacturers recommend starting suction prior to crossing the lesion during thrombectomy to minimise distal embolisation. Therefore, it is surprising that 20% of respondents start suction only after they have passed through the lesion. This suggests that further education and training is needed in clinical practice, and future clinical trials of thrombectomy need to ensure operators use the devices in an optimal fashion.
UPDATE OF CLINICAL TRIALS OF THROMBECTOMY DURING STEMI
The INFUSE AMI trial (n=452) demonstrated no difference in MRI infarct size in patients with anterior STEMI treated with aspiration thrombectomy compared to PCI alone5. Currently, there are two investigator-initiated large multicentre randomised trials evaluating thrombectomy during PPCI for STEMI that have started since this survey was performed: 1) the Thrombectomy With PCI Versus PCI Alone trial (TOTAL) which is being performed by our group (n=4,000, NCT01149044), and 2) the Thrombus Aspiration in STEMI in Scandinavia trial (TASTE) performed within the Swedish Angiography and Angioplasty registry (n=5,000, NCT01093404)6.
With regard to pharmacological therapy, our survey suggests that GP IIb/IIIa inhibitors appear to be more commonly used as a default strategy than bivalirudin. This is despite the HORIZONS-AMI trial which demonstrated reductions in bleeding and mortality with bivalirudin when compared to heparin with GP IIb/IIIa inhibitors7. In addition, vasodilators are being used to prevent no-reflow routinely by nearly one third of respondents despite the lack of evidence to support this practice.
Limitations
First, the generalisability of the survey results is probably limited by the distribution of the survey to operators from centres participating in clinical trials, whose practice may differ significantly from operators in other centres. Second, the response rate of 29% may have contributed to a non-response bias8. Several strategies were implemented to maximise response rate including minimising time for completion, ensuring relevance of all questions through a pilot survey, and follow-up e-mails. Physician-based surveys tend to have low response rates with rates between 13-50%9,10. Third, routine use was defined as the default approach, but actual percentages were not provided to participants to define routine use. Finally, survey results are self-reported and may differ from actual practice. It is important to note that respondents were experienced operators, the majority having more than 10 years of experience and performing more than 200 PCIs per year.
Conclusion
In contemporary practice, the majority of interventional cardiologists are not using aspiration thrombectomy routinely during PPCI. The vast majority of respondents stated that a large, definitive trial of aspiration thrombectomy was needed and this led to the design and start of the TOTAL trial (n=4,000). Finally, there is significant variability in the clinical use of evidence-based pharmacological therapies, direct stenting, and radial access during primary PCI.
Conflict of interest statement
S. Jolly, V. Dzavik, J. Cairns, and R. Welsh have received grant support from Medtronic. P. Widimsky has received speaker honoraria from Medtronic. S. Jolly has received honoraria from Bayer Interventional. All other authors have no conflicts of interest to declare.

