Abstract
Primary percutaneous coronary intervention (PPCI) has dramatically changed the scenario of ST-segment elevation myocardial infarction, consistently decreasing mortality and morbidity. These goals have been reached thanks to multiple technical and pharmacological refinements. The prevention of ischaemic complications via a combined pharmacoinvasive approach in these patients should concomitantly avoid bleeding events. While the focus in recent years has been on the relevance of bleeding complications, more recent data emphasise the need to optimise pharmacological treatment strategies in the very acute phase of intervention to minimise intraprocedural and early stent-related thrombotic events. The optimal treatment combination, including anticoagulant, oral and parenteral antiplatelet agents remains a matter of ongoing debate. In this paper we review the scientific basis of current era antithrombotic management during PPCI, trying to address some relevant questions, including the timing of initiation of antithrombotics and discussing available treatment options in the light of recent trial results.
Introduction
ST-segment elevation myocardial infarction (STEMI) management with primary percutaneous coronary intervention (PCI) has consistently evolved over time, along with different perceptions about the risks associated with ischaemic or bleeding events. In 2004, a patient with STEMI had to be treated according to existing guidelines with a glycoprotein IIb/IIIa inhibitor (GPI) with no emphasis on access-site selection, whereas recommendations today would rather favour a strategy of bivalirudin with the transradial approach. However, are these pillars standing on solid ground? Some recent research has instilled doubts. In addition, it seems that the continuous attempt to identify a “one size fits all” antithrombotic regimen for STEMI patients undergoing primary intervention is now obsolete. Yet, the fashionable motto according to which selection of antithrombotics should be based on bleeding and ischaemic risk remains problematic as it lacks real scientific prospective validation.
The evolution of antiplatelet and anticoagulant treatments (here called “antithrombotics”) over the last couple of decades has decreased the morbidity and mortality associated with STEMI1. Aspirin should be started rapidly, and subsequently low-dose daily aspirin is recommended for life; the addition of a P2Y12 inhibitor as dual antiplatelet therapy (DAPT) is advised early on and should be continued for nine to 12 months. In contrast, anticoagulation administered intravenously or subcutaneously and GPI are only recommended for hours/days in patients treated medically, and are discontinued just after the procedure or during the following hours in patients undergoing primary PCI.
The potential benefit of reducing ischaemic complications with an antithrombotic therapy has to be balanced with the risk of bleedings. Importantly, major bleedings have been associated with worse prognosis in randomised clinical trials and observational reports2. Whereas acute bleeding, especially intracerebral haemorrhage, can result in death, the premature interruption or cessation of an antithrombotic therapy can result in stent thrombosis, myocardial infarction (MI), peripheral embolisation, stroke, or death3. Therefore, many challenges need to be addressed to optimise the benefit/risk ratio with antithrombotics today.
Here we describe current antithrombotic management of STEMI patients focusing on the latest trials, and attempt to give a key to understanding some of the conflicting results of recently disclosed studies.
Anticoagulant and antiplatelet drugs administered before coronary angiography
Platelets play a central role in thrombus formation, and in the setting of STEMI there is a higher number of activated platelets, whose hyperreactivity is associated with a greater incidence of major adverse cardiac events (MACE)4.
The early administration of antithrombotic treatment seems particularly intriguing, especially in those areas where the catheterisation laboratory is not easily accessible and treatment delay is anticipated. In those patients who cannot benefit from rapid transportation to a cathlab, and where the time from first medical contact to primary PCI performed by an experienced team exceeds 120 minutes, administration of intravenous fibrinolytic agents is recommended by current guidelines1. This aspect is developed in a dedicated article of this issue.
Some studies have suggested a potential advantage of starting GPI at first medical contact, with angiographic and reperfusion improvements and infarct size reduction after primary PCI5-10. A post hoc analysis of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial, which tested the efficacy of pexelizumab in reducing reperfusion injury, showed that GPIs given prior to the catheterisation laboratory reduced 90-day death and death/congestive heart failure/shock without increasing bleeding11. Similar results were obtained in the web-based EUROTRANSFER registry12. The Facilitated INtervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) study was powered for comparing the combination of abciximab plus a low dose of reteplase (combo therapy) versus abciximab given in the cathlab in primary PCI patients in whom a considerable delay in treatment was to be expected. The study group receiving abciximab on the way to the catheterisation laboratory was not powered for. Hence, study results for timing of abciximab administration should be interpreted with caution. Despite an improvement in angiographic endpoints in patients receiving the combo therapy, cardiac adverse events at 90 days did not differ in the three groups13. Current recommendations from the European Society of Cardiology (ESC) suggest that GPI may be administered prior to the cathlab in selected high-risk patients, with a class IIb, level of evidence B, which is supported by the results of the Ongoing Tirofiban In Myocardial infarction Evaluation (ON-TIME 2) study1,14. Importantly, patients presenting with a short delay from onset of symptoms to tirofiban administration were those deriving the greatest benefit from treatment.
The ESC guidelines recommend an early use of P2Y12 inhibitors. Though there are limited data on the topic in relation to clopidogrel15, the newer P2Y12 inhibitors do not have studies assessing the potential value of pre-treatment in the primary PCI setting. The ongoing Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation myocardial Infarction to open the Coronary artery (ATLANTIC) trial is evaluating if a ticagrelor loading dose given in the ambulance is superior to periprocedural ticagrelor in terms of angiographic, reperfusion and safety endpoints16. The role of GPI pre-treatment should also be evaluated taking into consideration the more potent and faster onset of action of new P2Y12 inhibitors.
The recently published European Ambulance Acute Coronary Syndrome Angiography (EUROMAX) trial compared pre-hospital treatment with bivalirudin versus unfractionated heparin (UFH)±GPI, showing an improved primary outcome (death+major bleeding at 30 days) in the bivalirudin arm (5.1 vs. 8.4%, p=0.002) without differences in terms of death or MACE. However, there was a significant increase in acute definite stent thrombosis in the bivalirudin arm (1.1 vs. 0.2%, p=0.007). This observation came despite a post-procedural prolonged infusion of the drug in 93% of the patients (although with a minimum allowed infusion length of two hours)17. It remains to be established if the post-PCI bivalirudin regimen, i.e., full versus low regimen, is critical in preventing acute ST events.
In conclusion, current evidence does not support routine pre-hospital administration of new P2Y12 inhibitors or intravenous (IV) antithrombotics except for a selected group of patients at high risk of thrombotic complications, or where primary PCI cannot be performed in a timely fashion.
Anticoagulant and antiplatelet drugs administered in the cathlab
ORAL ANTIPLATELETS
New oral P2Y12 inhibitors have been shown to improve clinical outcomes when compared to clopidogrel. In the subgroup analysis of STEMI patients of the TRial to assess Improvement in Therapeutic Outcomes by optimising platelet inhibitioN with prasugrel-Thrombolysis In Myocardial Infarction (TRITON-TIMI 38 study), prasugrel given during PCI proved to be superior to clopidogrel regarding the primary endpoint of death/MI/stroke at 30 days (6.5 vs. 9.5%, p=0.0017) and there was a significant reduction in stent thrombosis (1.2 vs. 2.4%, p=0.0084). After 15 months, the primary endpoint was still superior in the prasugrel group. The rates of TIMI non-CABG-related major bleeding did not differ in the two study groups. Importantly, there was no signal for interaction between type of ACS and outcomes in TRITON18. Similarly, the STEMI patients recruited in the Platelet Inhibition and Patient Outcomes (PLATO) trial derived a similar degree of benefit as compared to the overall study population, suggesting that the benefit observed in the overall study population can also be extrapolated to STEMI patients. Importantly, despite the fact that the reduction of the primary endpoint of cardiovascular death, MI or stroke was not significant (9.4% vs. 10.8%, p=0.07), a consistent signal towards mortality reduction was observed in STEMI patients receiving ticagrelor as compared to clopidogrel (4.5% vs. 5.5%, p=0.07). In the PLATO as well as in the STEMI subpopulation, overall major bleeding did not differ in the two study groups (HR 0.98, p=0.76)19.
The advantages of new oral P2Y12 inhibitors are that they have a more potent as well as consistent platelet inhibition, and a faster onset of action. However, both prasugrel (Facilitation through Aggrastat By drOpping or shortening infusion line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse, FABOLUS PRO study) and ticagrelor have shown a considerable delay in providing full platelet inhibition in the first few hours after administration in STEMI patients20. Moreover, it has recently been observed that morphine administration in the pre-hospital setting might further reduce their onset of action21. This provides the opportunity to use intravenous antiplatelet agents to bridge the acute phase of intervention, in which the degree of platelet inhibition seems to be critical to optimise ischaemic outcomes. Indeed, the use of GPI has been considerable in both the TRITON and PLATO studies, with no interaction between use of GPI and allocated treatment in these two studies.
INTRAVENOUS ANTIPLATELETS
The role of GPIs in this setting has been investigated in multiple placebo-controlled studies, including Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up (ADMIRAL), Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC), Abciximab and Carbostent Evaluation (ACE), and the ONgoing Tirofiban In Myocardial infarction Evaluation (ON-TIME 2)14,26-28. However, their routine value in this setting has been questioned by the results of more recent investigations.
The Bavarian Reperfusion AlternatiVes Evaluation (BRAVE) 3 trial, which recruited 800 patients undergoing primary PCI treated with a 600 mg clopidogrel loading dose, randomised patients to abciximab or placebo. The GPI group had similar final infarct size at myocardial perfusion scan (study primary endpoint) and 30-day MACE29. The availability of new P2Y12 inhibitors, which shared consistent data and a faster onset of action if compared to clopidogrel, has also been regarded as an additional reason to drop the use of GPI. As discussed above, the most recent evidence shows a delay in onset of action of new P2Y12 inhibitors in the range of a few hours, especially in the setting of STEMI. Finally, a higher attention to bleeding complications that have been linked to an increased risk of mortality has played a pivotal role in dropping the use of GPIs.
An ongoing debate is whether the use of the transradial approach would obviate the need for bivalirudin to reduce bleeding events. A pooled data set of patients in the Randomised Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-2, Acute Catheterisation and Urgent Intervention Triage strategY (ACUITY), and Harmonizing Outcomes with Revascularisation and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trials who underwent PCI, including 17,393 patients, showed access-site bleeding occurring in 2.1% of all patients, whereas the rate of non-access-site bleeding was 3.3% overall30. Interestingly, bivalirudin was shown to reduce not only access-site but also non-access-site bleeding complications, which provides a possible rationale for the use of bivalirudin in patients in whom the transradial access site is used. Moreover, the adjusted one-year mortality risk of a non-access-site bleed was significantly greater than that of an access-site bleed (HR: 2.27, 95% CI: 1.42 to 3.64, p<0.0007). Therefore, this analysis would suggest that any bleeding is deleterious, yet those which are non-access-site-related may impact even more on cardiovascular outcomes31.
The ESC guidelines relegate GPI use during primary PCI to a bail-out treatment in case of high thrombus burden, thrombotic complications or no-reflow/slow-flow (class IIa), whereas their routine use may be considered in selected scenarios (class IIb)1. A critical analysis of the BRAVE 3 and ON-TIME 2 studies would suggest that GPIs may still have a role when the time from symptom onset to presentation is short14,29. This observation would fit with the current paradigm that early thrombus formation is highly dependent on platelet aggregation, whereas over time thrombus becomes more and more stabilised and becomes less responsive to intense platelet inhibition. Moreover, it remains likely that intense platelet inhibition may be particularly beneficial in patients with a short duration of myocardial ischaemia, a setting in which the benefit of preventing microvascular obstruction before, during and perhaps even after coronary vessel recanalisation may be magnified given the presence of a large amount of ischaemic, yet viable myocardium (Figure 1).
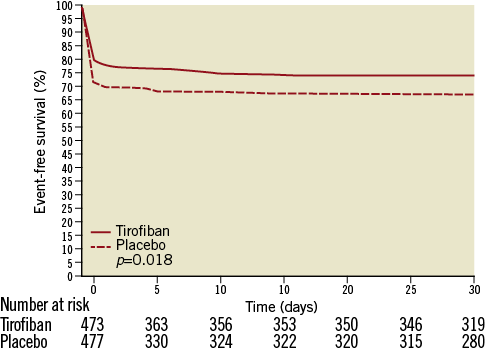
Figure 1. Kaplan-Meier event-free survival curves (death, recurrent myocardial infarction, urgent target vessel revascularisation, or blinded bail-out use of study drug) in patients treated with high bolus dose of tirofiban or placebo, from the ON-TIME 2 study. (Reproduced with permission14).
In this setting, the option to give an intravenous P2Y12 inhibitor, namely cangrelor, to bridge the onset of action of oral antiplatelet agents and optimise outcome is also intriguing. Cangrelor has been tested in the whole spectrum of PCIs in the Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) studies22-24. A pooled analysis of these trials showed an improvement in the primary outcome of death, MI, ischaemia-driven revascularisation or stent thrombosis at 48 hours as compared to a loading dose of clopidogrel 600 mg, and no signal of interaction has been noted between STEMI versus patients undergoing PCI for other indications25. However, this drug is currently not approved and, before suggesting its use during primary PCI, a broader clinical experience is warranted.
INTRAVENOUS ANTICOAGULANTS
Bivalirudin is a direct thrombin inhibitor and, differently from heparins, is able to inhibit both soluble and fibrin-bound thrombin with similar potency. Furthermore, heparins slightly potentiate platelet activation, whereas bivalirudin inhibits platelet aggregation. The HORIZONS-AMI was a prospective, multicentre randomised trial in 3,602 STEMI patients undergoing primary PCI. At 30 days, bivalirudin alone given for the duration of PCI demonstrated statistical superiority versus UFH plus GPI for the two primary endpoints of net adverse clinical outcomes (9.2% vs. 12.1%, p=0.006) and major bleedings (4.9% vs. 8.3%, p=0.0001). Treatment with bivalirudin also resulted in significantly lower 30-day rates of cardiac mortality (1.8% vs. 2.9%, RR=0.62, p=0.028) and all-cause mortality (2.1% vs. 3.1%, RR=0.66, p=0.047). However, patients treated with bivalirudin had a higher rate of acute stent thrombosis (1.3% vs. 0.3%, p<0.001)32.
The more recent EUROMAX study, where TRI and TFI were equally represented and the addition of GPI to UFH was left at the discretion of the treating physician, failed to show a mortality advantage with bivalirudin at 30 days. EUROMAX showed a reduction of major bleeding complications in the bivalirudin arm, which seems consistent with that observed in previous trials where the use of TRI was negligible and GPIs were protocol-mandated17.
Multiple studies have raised some issues with respect to the value of bivalirudin in preventing ischaemic events, including the occurrence of acute stent thrombosis in STEMI or the incidence of periprocedural MI in the setting of P2Y12 naïve NSTEACS patients (Figure 2). As GPI use in the comparator arm in all these studies was very high, a possible interpretation of these findings is that bivalirudin-treated patients, despite the drug’s capability to inhibit thrombin-dependent platelet activation, remain critically dependent on P2Y12 pathway inhibition in order to minimise the ischaemic risk. On the other hand, it has been suggested that prolonging bivalirudin infusion well after PCI may effectively and safely overcome the lack of adequate P2Y12 inhibition, while thereafter allowing a gradual bridging with oral P2Y12 inhibitors.
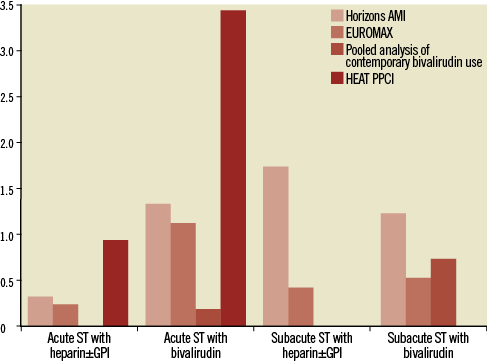
Figure 2. Early stent thrombosis rates across various studies with bivalirudin versus heparin±glycoprotein IIb/IIIa inhibitors. GPI: glycoprotein IIb/IIIa inhibitors; ST: stent thrombosis
However, in EUROMAX, despite protocol-mandated prolongation of bivalirudin after PCI, which was largely dosed at 0.25 mg/kg/hr, the incidence of acute ST remained higher in the bivalirudin arm17. Hence, it remains to be assessed if concomitant or early administration of more potent P2Y12 inhibitors and/or prolonged full regimen bivalirudin post-PCI infusion mitigates this risk.
The Bavarian Reperfusion AlternatiVes Evaluation (BRAVE) 4 tested the hypothesis that in primary PCI a strategy of prasugrel plus bivalirudin was superior to UFH plus clopidogrel in terms of the primary endpoint of 30-day MACE plus stent thrombosis, bleeding or stroke. This study was stopped after enrolment of only 548 patients instead of the planned 1,240 due to slow recruitment33. The single-centre open-label How Effective are Antithrombotic Therapies in PPCI (HEAT PPCI) trial34 enrolled 1,829 STEMI patients undergoing primary PCI and compared bivalirudin with no post-PCI infusion vs. UFH and a bail-out strategy of GPI. The primary endpoint of 28-day major adverse cardiovascular events was increased in the bivalirudin group (RR 1.52, CI: 1.1-2.1, p=0.01), key drivers being reinfarction and target lesion revascularisation, whereas bleeding events were similar. Moreover, in the bivalirudin group the rate of definite or probable acute/subacute stent thrombosis was significantly higher (3.4% vs. 0.9%, RR 3.91, p=0.001).
One mechanism that may explain the increased risk of acute ST in bivalirudin-treated patients is the rapid clearance of the drug after discontinuation at the end of PCI. An alternative mechanism related to an increase in stent thrombosis is a still inadequate platelet inhibition at the end of PCI when bivalirudin is discontinued.
The interpretation of the results of studies that involved bivalirudin is not straightforward. Therefore, a conclusive opinion on the use of this drug still requires further assessment in adequately powered studies. There is indeed heterogeneity in the global acceptance of this drug, which is much higher in the USA compared to European countries. The significant cost of the drug probably also plays a role.
Does the intracoronary route provide some advantages?
There are some theoretical advantages of intracoronary (IC) antithrombotic administration over the IV route, resulting in high local drug concentrations. If the evidence for the intracoronary use of tirofiban, eptifibatide and bivalirudin is currently limited to a few case series or underpowered studies, IC abciximab bolus administration has been widely investigated, especially in case of high thrombus burden. A very selective, intrathrombus way of administration showed interesting preliminary data in reducing thrombus burden35, and a more pronounced local inhibition of platelet function and a higher degree of GP IIb/IIIa receptor occupancy compared to a standard IV bolus injection (93.5% vs. 74.0%, p=0.04) has recently been reported36. Some pilot studies showed reduced microvascular obstruction and smaller infarct size with this route37-40. On the contrary, probably reflecting different study design and patient populations, meta-analyses recently published showed conflicting results regarding hard clinical endpoints with the IC route41-44.
There are currently two trials powered for hard clinical endpoints. The Abciximab Intracoronary versus intravenously Drug Application in STEMI (AIDA STEMI) trial randomised 2,065 patients to abciximab given IV or IC via the guiding catheter and found no clinical benefit in terms of the composite endpoint of death, reinfarction and heart failure, with a borderline reduction in the secondary endpoint of heart failure45. Differently from other studies, the AIDA MRI substudy showed no benefit on infarct size with the IC administration of abciximab46.
The INFUSE-AMI study enrolled patients with large anterior MIs within five hours from symptoms onset treated with bivalirudin and randomised 452 patients to IC abciximab delivered with a microcatheter directly inside the lesion or a placebo. IC abciximab was associated with reduced 30-day infarct size independently from manual thrombus aspiration47. Moreover, the recently published 12-month results showed a reduced composite endpoint of one-year death/severe heart failure/stent thrombosis, and a reduced death rate between one and 12 months48.
The better results of the INFUSE-AMI study might be explained by the superselective way of infusion of abciximab used in this study. When abciximab reaches very high local concentrations, it has been shown to disaggregate platelet aggregates and inhibit the production of tissue factor, a potent procoagulant35.
It would be interesting to address, in dedicated clinical trials, the synergy of manual thrombus aspiration with dedicated catheters, and subsequent very selective, intralesional infusion of GPIs via the same catheter, in order to optimise the use of commonly used devices, and reduce the costs for additional ones.
Antithrombotic regimen during the first hours after primary PCI
It is common practice to stop heparin after primary PCI unless clinically needed otherwise. A post hoc analysis of the CADILLAC trial showed that after coronary stenting a post-procedural infusion of heparin without GPI was associated with similar early or late MACE and higher bleedings49.
What is the role of a prolonged infusion of GPIs after primary PCI? Historically, abciximab infusion has always been continued for 12 hours and tirofiban and eptifibatide for at least 12-18 hours. However, the post-procedural infusion of this class of drugs is currently questioned. Christ et al, in a series of 56 patients on top of prasugrel (60 mg as bolus dose) or clopidogrel (600 mg), measured the effect of a single IC bolus of abciximab (0.25 mg/kg) on platelet aggregation, and found that overall platelet reactivity remained inhibited for at least 48 hrs, questioning the value of continuous abciximab infusion in this setting50. Similarly, the Facilitation through Abciximab By drOpping Infusion Line in patients Undergoing coronary Stenting - SYNergy with Clopidogrel at High loading dOse Regimen (FABOLUS SYNCHRO) study, which compared abciximab bolus-only versus abciximab followed by standard 12-hr infusion in a double-blind manner on the degree of platelet inhibition, found an identical degree of ADP or TRAP-induced platelet aggregation up to at least 24 hours in the two study groups51.
Hence, a bolus-only strategy seems particularly appealing when small-molecule GPIs are used. In the FABOLUS PRO study, a strategy of prasugrel loading dose was found insufficient to warrant adequate platelet inhibition after two hours when compared to tirofiban. Interestingly, patients treated with clopidogrel needed a post-procedural infusion of tirofiban to be able to suppress platelets effectively over time, whereas in patients treated with prasugrel a tirofiban bolus-only strategy obviated the need for subsequent infusion and almost completely abolished residual platelet reactivity (Figure 3)20.
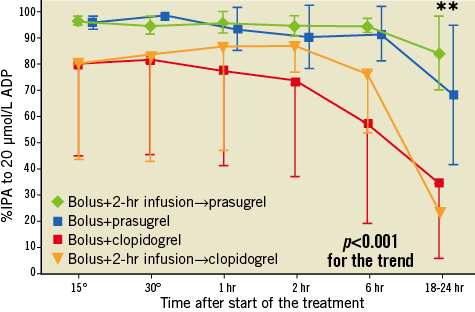
Figure 3. Percentage of platelet inhibition in patients treated with tirofiban, with or without infusion and with or without prasugrel or clopidogrel during and in the first hours after primary PCI, from the FABOLUS PRO study.
A prolonged infusion of bivalirudin after PCI has been tested in small-scale single-centre randomised trials52 and in multicentre retrospective registries53-56. Preliminary data from these pilot studies have suggested that prolonging bivalirudin infusion after PCI completion may lower the incidence of periprocedural MIs in elective, high-risk PCI, and the rate of acute stent thrombosis contemporarily improving reperfusion compared to intraprocedural infusion only.
Notably, a post hoc analysis of the EUROMAX study suggested that, in patients where a dose of 1.75 mg/kg/hr was used (22%), there was a similar rate of stent thrombosis as compared to patients treated with heparin (0.5% vs. 0.2%; p=0.45)57.
The ongoing Minimising Adverse haemorrhagic events by TRansradial access site and systemic Implementation of angioX (MATRIX) trial was designed to assess the impact of transradial versus transfemoral accesses and of bivalirudin versus UFH plus provisional GPI, on fatal and non-fatal ischaemic events58. This trial, which will enrol up to 8,500 patients in the whole spectrum of acute coronary syndromes undergoing early invasive management, will per protocol compare standard bivalirudin treatment to a prolonged post-PCI infusion. This study will thus provide unique randomised data regarding risks vs. benefits of a prolonged post-procedural bivalirudin infusion (Figure 4). However, the role of a prolonged infusion of bivalirudin should also take into consideration the increased final procedural cost.
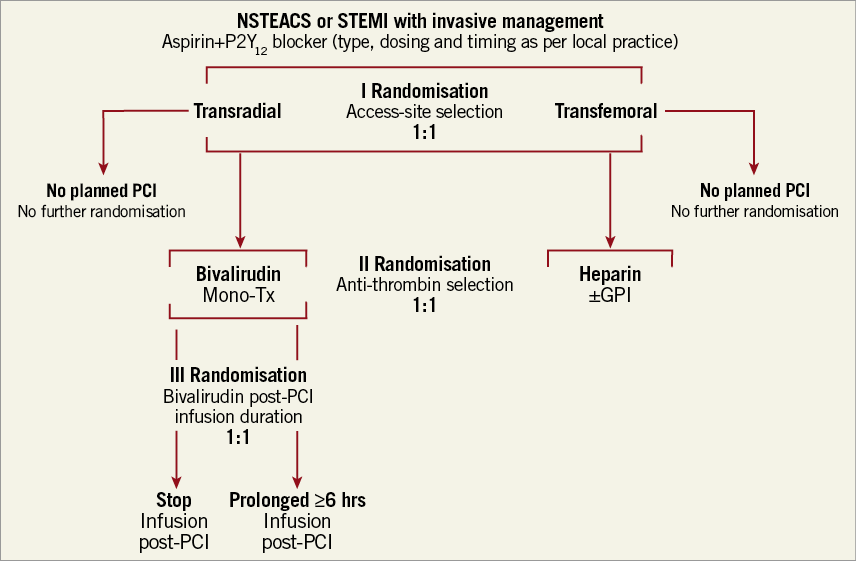
Figure 4. Flow chart of the ongoing MATRIX study. GPI: glycoprotein IIb/IIIa inhibitors; NSTEACS: non-ST-segment elevation acute coronary syndromes; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction; Tx: therapy
Critical/open issues and future perspectives
The short-term mortality of STEMI patients has progressively decreased in recent decades, also thanks to progressive refinements of the so-called pharmacoinvasive approach. Multiple antithrombotic treatment combinations have been tested in the setting of primary PCI patients and it has become progressively clear that clinicians and interventional cardiologists have the difficult task of tailoring their use based upon the perceived ischaemic versus bleeding risk of the individual patients. While both ischaemic and bleeding scores have been proposed to predict outcomes, their use in the setting of primary PCI patients remains problematic, as the decision with respect to which antithrombotic regimen to choose has to be performed before these scores can be computed. Moreover, covariates predicting ischaemic risk frequently overlap with those used to forecast bleeding risk status, and their performance is frequently perceived as suboptimal.
The MATRIX trial is currently investigating the value of bivalirudin versus UFH followed by provisional GPI in contemporary ACS patients who will be treated via either the transfemoral or the transradial access site as per randomisation scheme. The ATLANTIC study is investigating the value of starting ticagrelor in the pre-hospital setting in terms of both efficacy and safety, whereas the HORIZONS II AMI study will address the role of cangrelor on top of bivalirudin in primary PCI patients. The results of these studies are expected to shed new light on the best treatment combination or timing of administration with respect to the multiple treatment options which have become available in current clinical practice.
Conflict of interest statement
B. Cortese reports having served as consultant for The Medicines Company and AstraZeneca. M. Valgimigli has received fees for lecturing and is on the advisory board for The Medicines Company, AstraZeneca and Correvio International. R. Sebik has no conflicts of interest to declare.

