Introduction
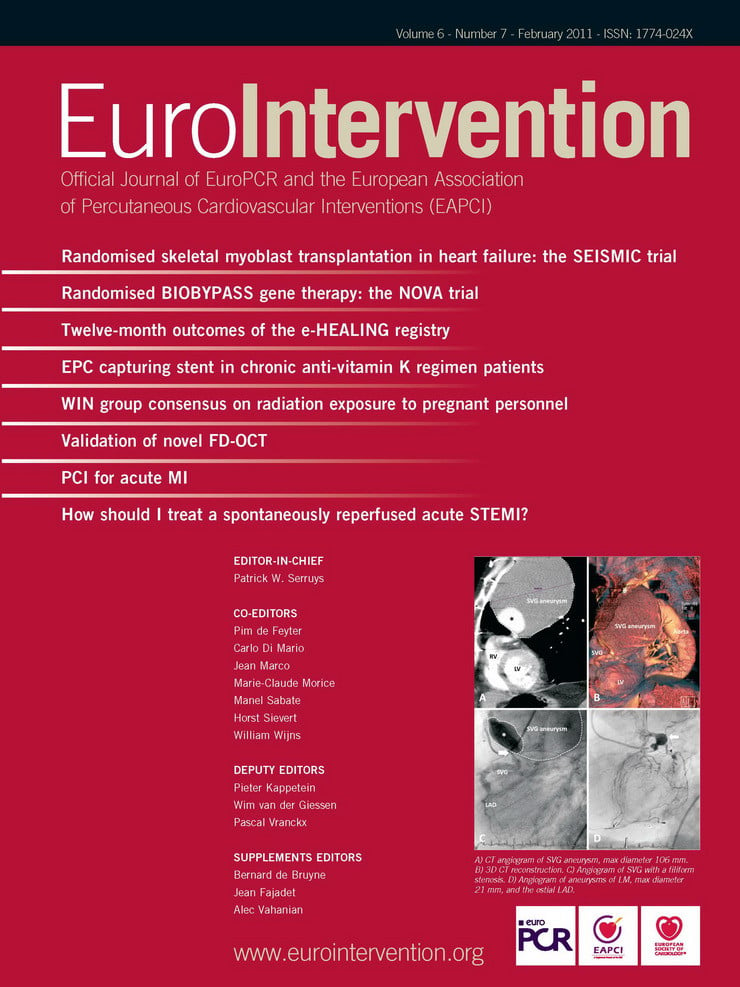
A myocardial infarction (MI) is usually caused by a sudden thrombotic obstruction of a coronary artery, superimposed on a ruptured atherosclerotic plaque. The location and duration of the occlusion, as well as the extent of collateral circulation are the main determinants of infarct size, whereas infarct size itself is a major determinant of prognosis. In animals with a coronary collateral circulation similar to that of humans an occlusion that persists for less than 30 min generally does not lead to permanent myocardial damage.1 Longer durations of coronary occlusion result in progressive growth of the infarction (Figure 1).
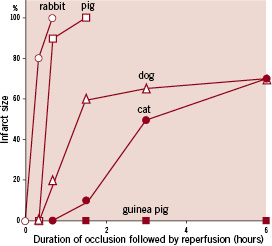
Figure 1. Development of infarct size as percentage of the infarct size that would occur when the coronary artery is permanently occluded in various animal species (Figure adapted and modified from reference 1).
The amount of salvageable myocardium rapidly decreases in the 30-120 min period after onset of the occlusion. If the occlusion persists for six hours, the infarct zone will reach its full size.
The evolution of infarct size over time in humans was nicely described in an early study of 1,334 MI patients who received alteplase.2 Infarct size was estimated from the cumulative release of the enzyme alpha-hydroxybutyrate dehydrogenase in plasma during 72 hours (α-HBDH72). The cumulative enzyme release was small in patients who were treated within one hour after onset of symptoms (Figure 2).
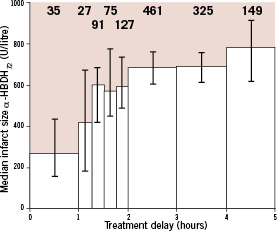
Figure 2. Effect of delay of fibrinolytic treatment on infarct size. Numbers above columns represents number of patients. Columns represent mean values; vertical bars represent 95% confidence intervals. α-HBDH72 equals the cumulative activity of myocardial alpha-hydroxybutyrate dehydrogenase released per litre of plasma during the first 72 hours after acute myocardial infarction. (Figure adapted and modified from reference 2)
A steep increase in α-HBDH72 was observed when treatment was initiated between one and two hours after symptom onset, whereas α-HBDH72 only slightly increased with increasing treatment delays beyond two hours. This pattern indicates that viable myocardial muscle cells are rapidly and massively lost in the period early after the onset of the coronary occlusion.
Insight in these pathophysiological mechanisms has opened therapeutic windows. Since the early 1980s, treatment strategies have been introduced that aim at a rapid, complete and persistent restoration of the coronary blood circulation to avoid myocardial cell damage. These strategies are either based on a pharmacological intervention (fibrinolytic therapy, often combined with antiplatelet and antithrombin treatment), a mechanical intervention (coronary angioplasty, with or without stent placement) or their combination. This review intends to summarise the key findings from clinical trials that were undertaken during 1980-2009 to evaluate the effectiveness and safety of these options. It will appear that strategies including a percutaneous coronary intervention (PCI) produced the most favourable clinical outcome in these trials.
Fibrinolytic therapy
The value of fibrinolytic therapy in patients with evolving MI is well documented. Timely fibrinolytic therapy resolves coronary thrombi and terminates the process of myocardial necrosis, which results in preservation of viable myocardium and left ventricular function, and consequently, improved survival. In a meta-analysis of the 22 randomised trials of fibrinolytic therapy versus control that were reported during 1983-1993, fibrinolysis was associated with a 25% proportional reduction in short-term mortality (most trials reported events until 30-days after randomisation, a few trials had 35-day follow-up) in patients presenting with ST-elevation or new bundle branch block within 12 hours after symptom onset (number of patients [N] 50,246; 9.1% versus 11.9% events; odds ratio [OR] 0.75 and 95% confidence interval [CI] 0.70-0.80; p-value <0.001).3 This translates into an absolute mortality reduction of 27 (standard deviation [SD] 3) per 1,000 patients treated.
The proportional as well as the absolute mortality reduction by fibrinolytic therapy are strongly related to the time that has expired since the onset of symptoms, which is supposed to coincide with the moment of coronary occlusion. The proportional mortality reduction in the group of patients presenting within one hour after symptom onset was as high as 48% (OR 0.52 and 95% CI 0.39-0.69), whereas the absolute mortality reduction was estimated at 65 (SD 14) per 1,000 patients treated (Figure 3).3
Figure 3. Relation between time from onset of symptoms to randomisation and short-term mortality in 22 clinical trials of fibrinolytic therapy versus control. The grey-shaded bars in the left panel indicate patients who were randomised to fibrinolytic therapy, the open bars indicate patients who were randomised to control therapy. Most trials reported events until 30-days after randomisation, a few trials had 35-day follow-up.
Clearly, to realise the full potential of the lifesaving effects of fibrinolytic therapy, treatment should be initiated as soon as possible after symptom onset.
Fibrinolysis was also associated with a small, but significant increased risk of stroke (N 58,600; 1.16% versus 0.76% events; OR 1.52 and 95% CI 1.29-1.80; p-value <0.001).4 This excess of four (SD 1) extra strokes per 1,000 patients treated was largely attributed to intracranial haemorrhage (ICH). It should be noted that two of the excess strokes associated with fibrinolysis were fatal, and were already accounted for in the mortality figures.
During the 1980s and early 1990s multiple fibrinolytic treatment strategies have been developed and tested. These strategies are either based on so-called non-fibrin-specific (e.g., streptokinase) or fibrin-specific (e.g., alteplase, reteplase) agents, which are combined with antiplatelet (aspirin, platelet glycoprotein [GP] IIb/IIIa inhibitors) and antithrombin (unfractionated heparin [UFH], or heparin derivatives) therapy. In 1993, the GUSTO-1 trialists demonstrated a 15% proportional 30-day mortality reduction by ‘accelerated’ alteplase (100 mg infusion over 90 min, with over half of the dose within 30 min) over streptokinase in patients presenting with ST-elevation (N 30,600; 6.3% versus 7.3% events; OR 0.85 and 95% CI 0.78-0.94; p-value <0.001).5 Since then ‘accelerated’ alteplase became the standard for pharmacological reperfusion therapy.
During the 1990s several wild-type alteplase mutants were developed with a longer half-life, so that these agents could be administered via bolus injection. In a combined analysis of the GUSTO-3 (reteplase),6 COBALT (double bolus alteplase),7 ASSENT-2 (tenecteplase) and InTIME-2 (lanoteplase) randomised trials,8,9 ST-elevation patients who were randomised to bolus fibrinolytic agents had similar 30-day mortality as patients randomised to ‘accelerated’ alteplase (N 54,200; 7.0% versus 6.8% events; OR 1.04 and 95% CI 0.97-1.11; p-value 0.28). The bolus agents that were evaluated in COBALT and InTIME-2 were associated with an excess of four (SD 1) extra ICHs per 1,000 patients.7,9 In contrast, in ASSENT-2, tenecteplase was associated with a significant reduction of the risk of major bleeding complications.8 Taken the data of randomised trials together (N=103,972), meta-analysts suggested that bolus treatment was associated with an increased risk of ICH compared with infusion administration of the same agent (OR 1.75 and 95% CI 1.32-2.33; p-value <0.001), but this finding was challenged by other investigators, based on the same data10,11.
Percutaneous coronary intervention
The time that has expired between the onset of symptoms and the start of reperfusion therapy is an important, but not the single determinant of patient outcome. Additional factors are adequate restoration of coronary blood flow, restoration of microvascular and myocardial perfusion and the prevention of coronary reocclusion. Angiographic studies have shown that coronary reperfusion does not occur in 20% to 45% of patients receiving fibrinolytic treatment.12 In addition, 5% to 30% of patients may experience early or late reocclusion.13,14 Another disadvantage of the use of fibrinolytic agents is its associated risk of major, life-threatening bleeding complications. These facts have acted as a driving force to introduce percutaneous coronary intervention (PCI) as an alternative approach to reopen the occluded coronary artery.
Angiographic success rates of PCI in ST-elevation acute coronary syndromes (ACS) appeared to be as high as 90%.10,15 Several clinical trials demonstrated that these excellent angiographic results had significant positive effects on clinical outcome. In a meta-analysis of 22 randomised trials that were reported during 1993 and 2002, ‘primary’ PCI (i.e., PCI without systematic pharmacological pretreatment aiming at coronary reperfusion) was associated with a 30% proportional reduction in 30-day mortality compared to fibrinolytic therapy in patients presenting with ST-elevation within 12 hours after onset of symptoms (N=7,400; 5.3% versus 7.4% events; OR 0.70 and 95% CI 0.58-0.85; p-value <0.001).16 The absolute mortality was estimated at 21 (SD 6) per 1,000 patients treated. Furthermore, primary PCI was associated with an important, 66% proportional reduction in the 30-day incidence of non-fatal myocardial re-infarctions (N=6,500; 2.5% versus 6.8% events; OR 0.34 and 95% CI 0.27-0.45; p-value <0.001), and a 61% reduction in total stroke (N 6,000; 0.84% versus 2.11% events; OR 0.39 and 95% CI 0.25-0.62; p-value <0.001). The stroke reduction was largely due to a reduction in the incidence of ICH (0.06% versus 1.12% events). Altogether, primary PCI was associated with 62 (SD 8) fewer deaths, non-fatal reMIs or strokes per 1,000 patients treated.
These data clearly demonstrate that primary PCI is associated with better clinical outcome than fibrinolytic therapy. Still, primary PCI is not without limitations. First, it should be realised that the results of primary PCI are highly dependent on the experience of the operator and the interventional team. Evidence exists that operators should treat at least 75 patients per year in a centre in which the annual number of PCI procedures for MI amounts at least 200 in order to maintain high-level professional skills.17 However, the main challenge of primary PCI is the treatment delay that is involved in mobilising the interventional team and preparing the interventional facility. Under optimal circumstances, this will lead to a 30 min additional treatment delay as compared with fibrinolytic therapy, but usually PCI-related treatment delays are much longer.18
There is no evidence that the mortality reduction by primary PCI as compared to fibrinolysis is modified by the time from symptom onset to presentation. In a recent meta-analysis that was based on the individual patient data (N=6,700), no heterogeneity was observed in the proportional reduction in the odds of death by primary PCI in relation to presentation delay (Figure 4).19
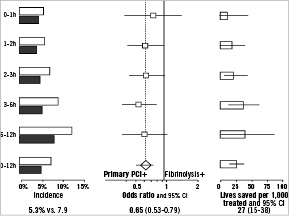
Figure 4. Relation between time from onset of symptoms to randomisation and 30-day mortality in 22 clinical trials of primary percutaneous coronary intervention versus fibrinolytic therapy. The grey-shaded bars in the left panel indicate patients who were randomised to primary percutaneous coronary intervention, the open bars indicate patients who were randomised to fibrinolytic therapy.
However, the results of the CAPTIM trial which showed that ST-elevation myocardial infarction patients randomised within two hours of symptom onset to fibrinolysis in stead of primary PCI had a strong trend towards lower 30-day mortality rates, were not taken into account in this meta-analysis.20 The WEST trial data suggested that the rates of cardiac events doubled if patients were treated with primary PCI beyond two hours of symptom onset compared to mechanical reperfusion within two hours, however no specific data on mortality in relation to presentation delay was available for fibrinolysis and primary PCI.21 All tabulated data together of published randomised trials (N=8,000) showed that PPCI decreased 30-day mortality rates compared to fibrinolysis, regardless of presentation delay ([≤2 hours] OR 0.84 and 95% CI 0.61-1.17; [≥ 2hours] OR 0.67 and 95% CI 0.54-0.84), although this difference was most noticeable for patients treated after two hours of symptom onset.
Still, in that meta-analysis, the proportional mortality reduction of primary PCI compared to fibrinolysis was dependent on the additional treatment delay that was introduced by the more invasive approach. In particular, in clinical environments with procedure-related delays that are limited to a maximum of 35 minutes, primary PCI was associated with a 67% reduction in 30-day mortality as compared to fibrinolytic therapy (N=1,400; 2.8% versus 8.2% events; OR 0.33 and 95% CI 0.19-0.55; p-value <0.001). This translates into an absolute mortality reduction of 53 (SD 12) per 1,000 patients treated. In clinical environments with prolonged PCI-related delays (up to 120 minutes), primary PCI was associated with a 26% proportional mortality reduction (N=5,300; 5.9% versus 7.9% events; OR 0.74 and 95% CI 0.60-0.91; p-value 0.005), and an absolute mortality reduction of 19 (seven) per 1,000 patients treated.19 Thus, similar to fibrinolysis, increasing treatment delays diminish the lifesaving effects of primary PCI.
Facilitated percutaneous intervention
Facilitated PCI refers to a strategy of planned immediate PCI after administration of an initial pharmacological regimen that is installed to improve coronary patency before the procedure. These regimens have included platelet glycoprotein (GP) IIb/IIIa inhibitors, full-dose or reduced-dose fibrinolytic therapy, and the combination of a GP IIb/IIIa inhibitor with a reduced-dose fibrinolytic agent. The facilitated PCI strategies are designed to profit from the best of two worlds: rapid clot lysis (or at least prevention of further blood clotting) by means of a pharmacological agent, followed by complete and sustained revascularisation by subsequent coronary angioplasty.
Facilitation by glycoprotein IIb/IIIa inhibitors
The use of GP IIb/IIIa inhibitors in ST-elevation patients undergoing PCI inhibits platelet aggregation at the site of plaque rupture and procedure-induced injury, potentially improving the clinical outcome. Between 1998 and 2003 a total of eight randomised trials have studied the effectiveness and safety of the GP IIb/IIIa inhibitor abciximab versus control therapy. In a meta-analysis of these trials, abciximab was associated with a 30% proportional reduction in the incidence of 30-day mortality, although statistical significance at the conventional 0.05 level was not reached, most likely due to a lack of statistical power (N=3,900; 2.3% versus 3.3% events; OR 0.70 and 95% CI 0.48-1.02; p-value 0.062).22 A statistically significant 47% proportional reduction was observed in the incidence of (fatal or non-fatal) reMI (N=3,900; 0.99% versus 1.86% events; OR 0.53 and 95% CI 0.30-0.92; p-value 0.021). All trials together, there was no evidence of an increased risk of stroke or ICH by abciximab.
In view of these results, the use of GP IIb/IIIa inhibitors (particularly abciximab) is clinically indicated in ST-elevation patients undergoing primary PCI, similar to ACS patients presenting without ST-elevation. The question whether or not there is a beneficial effect of GP IIb/IIIa inhibitors that are administered as a facilitation agent to patients undergoing PCI has been addressed in 13 randomised trials that were reported between 2002-2009.23-27 In these trials, all patients received a GP IIb/IIIa inhibitor, but the timing of the administration was randomised. Patients who were randomised to early (i.e., as soon as the diagnosis ST-elevation ACS has been established) treatment with a GP IIb/IIIa inhibitor did not have reduced 30-day mortality compared to those who were randomised to treatment with a GP IIb/IIIa inhibitor at the time of the PCI procedure (N=2,800 ; 4.5% versus 3.9% events; OR 1.16 and 95% CI 0.80-1.69; p-value 0.44). There was also no statistically significant difference in the incidence of non-fatal reMI (1.48% versus 0.75% events), nor in the incidence of total stroke (0 versus 0.37% events). It should be realised, however, that the number of patients who were investigated in these trials is too small to exclude clinically relevant differences with sufficient certainty. Based on all trials with GP II/IIIa inhibitors in ACS, practical treatment guidelines recommend to administer abciximab as early as possible in patients with ST-elevation undergoing PCI.28
Facilitation by a (reduced dose) fibrinolytic agent
Between 1992-2009, seven randomised trials were reported that studied the effectiveness and safety of PCI facilitated by a (reduced dose) fibrinolytic agent with primary PCI.23,29 Based on all available evidence from these trials, we must conclude that facilitation by a fibrinolytic agent does not result in improved short-term mortality rates compared with primary PCI (N=5,800; 4.8% versus 5.3%; OR 0.91 and 95% CI 0.72-1.15; p-value 0.6) (Figure 5).
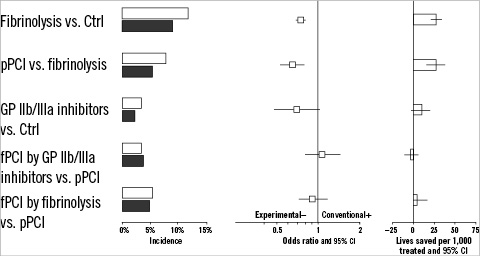
Figure 5. The comparison of several treatment modalities for acute myocardial infarction in terms of incidence, 30-day mortality OR and amount of lives saved per 1,000 patients treated. The grey-shaded bars in the left panel indicate patients who were randomised to experimental therapy, the open bars indicate patients who were randomised to control therapy.
Facilitated PCI by a fibrinolytic agent was also associated with an almost doubled risk of nonfatal reMI (N=2,900; 4.4% versus 2.4% events; OR 1.90 and 95% CI 1.25-2.89; p-value 0.002), and with a more than 5-fold increased risk of stroke (N=3,000; 1.57% versus 0.27% events; OR 5.91 and 95% CI 2.04-17.1; p-value <0.001).
The few randomised trials that studied the effectiveness and safety of PCI facilitated by a combination of GP IIb/IIIa inhibitors and a fibrinolytic agent showed similar negative results.22,24 Thirty-day mortality was increased by 31% in patients randomised to the “combined-facilitation” strategy versus those randomised to primary PCI (N=2,000; 4.9% versus 3.8% events; OR 1.31 and 95% CI 0.85-2.01; p-value 0.22).
Concluding remarks
After its introduction in the clinical arena in 1977,30 percutaneous transluminal coronary angioplasty was only performed in easily accessible proximal lesions of stable coronary patients with preserved left ventricular function. Thirty years later, PCI has a much broader indication. Due to increasing experience, the introduction of low-profile ‘steerable’ balloon catheters, the use of bare metal stents to avoid recoil of the artery, the use of ‘coated’ stents that release antiproliferative drugs to interfere with the process of restenosis, and due to optimised adjunctive medications to prevent thrombotic complications (aspirin, UFH, low-molecular weight heparins, direct thrombin inhibitors, clopidogrel, GP IIb/IIIa inhibitors, and factor Xa inhibitors), PCI has evolved into an effective and safe instrument to save the lives of patients in extremely unstable conditions. This brief overview of clinical trial results clearly demonstrates that PCI should be considered the first treatment of choice in patients presenting with evolving MI. Still, the “real world” poses formidable logistical and economic challenges to the feasibility of such a “PCI-for-all” approach. Therefore, one of the key aims for clinical cardiology today should be to implement effective actions, including pre-hospital diagnostic services, and 24-hour/7-day access to tertiary (regional) heart centres, that enable the delivery of this life-saving treatment in a timely fashion for all eligible patients.