
Abstract
Aims: The aim of this meta-analysis was to compare the benefit of “early” vs. “delayed” P2Y12 inhibition in patients undergoing percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI).
Methods and results: We conducted a meta-analysis including seven randomised controlled trials (RCTs) which compared early vs. delayed P2Y12 inhibition in STEMI patients scheduled for PCI, providing data on major adverse cardiac events (MACE), all-cause death, and major bleeding. The primary endpoint was MACE. Secondary endpoints included stent thrombosis and the use of GP IIb/IIIa inhibitors (GPI). All endpoints were analysed at the shortest follow-up available. A total of 9,648 patients were included (“early”=4,792, “delayed”=4,856). “Early” P2Y12 inhibition was associated with a significant reduction in MACE rate (OR 0.73, 95% CI: 0.61-0.88, p=0.0008), myocardial infarction (OR 0.71, 95% CI: 0.57-0.90, p=0.004), bail-out GPI use (OR 0.87, 95% CI: 0.75-1.00, p=0.04) and improved coronary reperfusion before PCI (OR for Thrombolysis In Myocardial Infarction [TIMI] flow grade 2-3=1.12, 95% CI: 1.00-1.26, p=0.04). Major bleeding was not increased (OR 0.87, 95% CI: 0.62-1.21, p=0.41).
Conclusions: A strategy of early effective P2Y12 inhibition in PCI of STEMI appears to improve coronary reperfusion before PCI, and reduce MACE, MI and bail-out GPI use without increase of major bleeding.
Abbreviations
CV: cardiovascular
GPI: glycoprotein IIb/IIIa inhibitors
MACE: major adverse cardiac events
MI: myocardial infarction
PCI: percutaneous coronary intervention
RCT: randomised controlled trial
STEMI: ST-elevation myocardial infarction
ST: stent thrombosis
TIMI: Thrombolysis In Myocardial Infarction
Introduction
STEMI is a highly thrombotic situation that needs rapid reperfusion and potent platelet inhibition. Previous studies have shown that mortality increases with delays to PCI exceeding 60 minutes after the first medical contact1, and that poor platelet inhibition increases the risk of recurrent ischaemic events2. Clopidogrel was the first P2Y12 inhibitor to show an effect on ischaemic events in secondary PCI of STEMI3. More recently, more potent and fast-acting P2Y12 inhibitors have demonstrated superiority over clopidogrel in PCI of STEMI4-6. Current European guidelines state that P2Y12 inhibitors are recommended before PCI of STEMI, or at the latest at the time of PCI (class of recommendation I, level of evidence A), reflecting the lack of certainty in the exact timing of P2Y12 inhibitor administration7.
The concept of early antiplatelet therapy during patient transfer to the catheterisation laboratory was first investigated with glycoprotein IIb/IIIa inhibitors (GPI), showing improved reperfusion and reduced ischaemic event rates, in particular for patients presenting early8.
Pretreatment with clopidogrel, while applicable for many clinical situations, is not appropriate for primary PCI of STEMI; due to its slow onset and low magnitude of effect, platelet inhibition is not obtained in time9. Prasugrel and ticagrelor are more potent and more rapidly acting than clopidogrel, but a delay of action of one to two hours is necessary to obtain significant platelet inhibition10. A strategy of early administration of these drugs in STEMI patients is compatible with a partially effective P2Y12 inhibition at the time of PCI. Finally, cangrelor is more potent than the oral drugs with an immediate onset of action after intravenous administration: it has the potential to provide a fully effective P2Y12 inhibition at the time of PCI11. For these reasons, we decided to compare in STEMI patients a strategy of early effectiveness of P2Y12 inhibitors (that sought to produce effective P2Y12 inhibition as rapidly as possible) compared with a strategy of effectiveness delayed to after PCI (that did not rely on the potential benefits of a more rapid effectiveness obtained with an earlier administration, or with the use of more rapidly acting drugs).
Methods
This meta-analysis was performed and reported according to PRISMA standards12. Details of the protocol for this systematic review were submitted for acceptance on PROSPERO (but not yet registered).
DATA SOURCES AND SEARCHES
The search included the following combination of terms: “PCI” AND “ST-elevation myocardial infarction” AND “clopidogrel” OR “prasugrel” OR “ticagrelor” AND “pre-treatment”, OR “loading” OR “timing” OR “upstream” OR “pre-hospital”, with no language restriction; for IV drugs, the search was enlarged to “PCI” AND “cangrelor” OR “elinogrel”. MEDLINE, Cochrane Controlled Trials Register, EMBASE and BioMed Central databases were scrutinised from January 1980 up to July 2017. Additional searches were also performed in Google Scholar, the medical websites tctmd.com, cardiosource.com and clinicaltrials.gov. Full-text articles and meeting abstracts of randomised controlled trials and pre-specified subgroups of randomised studies reporting data on P2Y12 inhibitor administration in STEMI were considered for analysis.
STUDY SELECTION
Titles and abstracts were reviewed and two independent reviewers (A. Bellemain-Appaix and G. Montalescot) evaluated full text articles for inclusion. Discrepancies were adjudicated by consensus. We included all studies published or presented at major meetings, with no language restriction, that met all of the following inclusion criteria: 1) randomised controlled trials including STEMI patients scheduled for PCI; 2) controlled comparison between early and delayed administration of a P2Y12 inhibitor or between two different P2Y12 inhibitors of different onset of action; 3) control arm corresponding to the administration of an oral P2Y12 inhibitor in the catheterisation laboratory at the time of PCI (no effective platelet inhibition during PCI); 4) data available on the type of P2Y12 inhibitor treatment, dose and timing of administration; and 5) data available on at least major adverse cardiac events (MACE), all-cause death, and major bleeding.
Exclusion criteria were: 1) studies where patients could have received any P2Y12 inhibitor before randomisation; and 2) all duplicate reports and ongoing studies.
DATA EXTRACTION AND QUALITY ASSESSMENT
Data extraction from published reports was carried out independently by two authors (A. Bellemain-Appaix and C. Bégué). Outcomes were reported at the shortest follow-up available in each study. The “early strategy” of P2Y12 inhibitor administration was defined as follows: i) administration of the drugs before arrival of the STEMI patients in the catheterisation laboratory (i.e., in the ambulance or in the emergency department or at a referring hospital), in comparison with the same drugs administered after arrival in the catheterisation laboratory (delayed strategy) or, ii) administration in the catheterisation laboratory before PCI of rapidly acting P2Y12 inhibitors (i.e., intravenous P2Y12 inhibitors or prasugrel or ticagrelor) in comparison with clopidogrel used in the control arm (delayed strategy). Late effectiveness always corresponded to the late administration of oral drugs in the catheterisation laboratory or after PCI.
The risk of bias was assessed independently by two reviewers (A. Bellemain-Appaix and C. Bégué), using the tool recommended and developed by the Cochrane Collaboration13. Disagreements were resolved through discussion.
DATA SYNTHESIS AND ANALYSIS
The primary efficacy endpoint was MACE according to each study definition at the shortest follow-up available (Supplementary Table 1). The primary safety endpoint was major bleeding according to the definition of each study (Supplementary Table 2). Secondary clinical endpoints included all-cause death, stent thrombosis (definite, according to the Academic Research Consortium definition), cardiovascular (CV) death, myocardial infarction according to each study definition (Supplementary Table 2), stroke, urgent target vessel revascularisation, minor and any bleeding. Additional surrogate endpoints were analysed when available: Thrombolysis In Myocardial Infarction (TIMI) 2-3 flow rate before and after PCI, ST-segment elevation resolution on the ECG before and after PCI, and the use of GPI. The corresponding authors of RCTs were contacted and asked to provide additional information not available in the literature: 1) in the TRITON-STEMI study4, only patients randomised at the time of PCI and who received P2Y12 inhibitors during or after PCI (72% of the population) were considered (for primary and secondary PCI) with a randomised comparison between prasugrel and clopidogrel; secondary PCI patients who may have received both drugs hours or days before reaching the catheterisation laboratory were excluded; 2) in the CHAMPION studies14,15, subgroup level data for STEMI patients were pooled by the authors; administration of cangrelor defined the “early” group (immediate efficacy), clopidogrel the “delayed” one; 3) the PLATO-STEMI study6 included 44% of patients treated with clopidogrel before randomisation; unpublished data on the other patients could not be obtained from the authors; the study was thus excluded from the main analysis, but we provide informative analysis integrating PLATO published data on MACE (without patients pre-treated), and the other endpoints (including patients pre-treated).
We conducted a study-level meta-analysis to give pooled estimates for each outcome. To avoid underweighting of some populations, studies were pooled using the Mantel-Haenszel fixed-effect model; a random-effects model was also tested as assuming the between-trial variance. The extent of heterogeneity between trials was assessed with Cochran’s Q test (with a cut-off p-value of 0.1 considered as significant for heterogeneity), and the I2 test for heterogeneity between subgroups was reported in each figure. Probability values were two-tailed with p=0.05 considered as significant. Odds ratios with 95% confidence intervals (CI) were calculated with the RevMan software version 5.0 (The Cochrane Collaboration) and the R meta-package (R Foundation for Statistical Computing, Vienna, Austria).
The main analysis was performed on all RCTs for the entire group of STEMI patients undergoing PCI. Several sensitivity analyses were performed to help to address the heterogeneity of study drugs and delay of administration according to: (1) the route of administration: IV vs. oral; cangrelor is the only available IV drug and, as opposed to oral drugs, has an immediate and potent effect; (2) the type of drug: clopidogrel vs. ticagrelor/prasugrel vs. cangrelor/elinogrel, that have a potency and onset of action gradually more favourable than clopidogrel; another analysis between clopidogrel and new P2Y12 inhibitors (all the others) was also performed; and 3) primary vs. secondary PCI, for which the time factor allows a longer delay to obtain effective P2Y12 inhibition. We also performed a stepwise influential analysis to identify trials that could have significantly driven the pooled effect.
Results
Finally, the search resulted in seven RCTs being selected (eight studies with fusion of STEMI data from the two CHAMPION studies) with a total of 9,648 STEMI patients (4,792 with an early and 4,856 with a delayed strategy of P2Y12 inhibition) (Supplementary Figure 1)3,4,14-19. Of the 9,648 patients, 6,694 received an oral drug (clopidogrel, ticagrelor or prasugrel), 6,914 were primary PCI patients, and 7,282 received a new P2Y12 antagonist (ticagrelor, prasugrel, cangrelor, or elinogrel). Details of the studies are provided in Supplementary Table 1. The overall risk of bias of the included studies never exceeded 25% (Supplementary Figure 2). No publication bias was observed, with a linear regression test of funnel plot asymmetry (p=NS for all explored outcomes).
MAIN ANALYSIS
MACE were reported at 30 days for six studies3,4,15-18 and at 48 hours for two studies14,19. The early inhibition strategy was associated with a significant reduction in the MACE rate with a fixed-effect model (OR 0.73, 95% CI: 0.61-0.88, p=0.0008), and confirmed by the random-effects model (Figure 1).
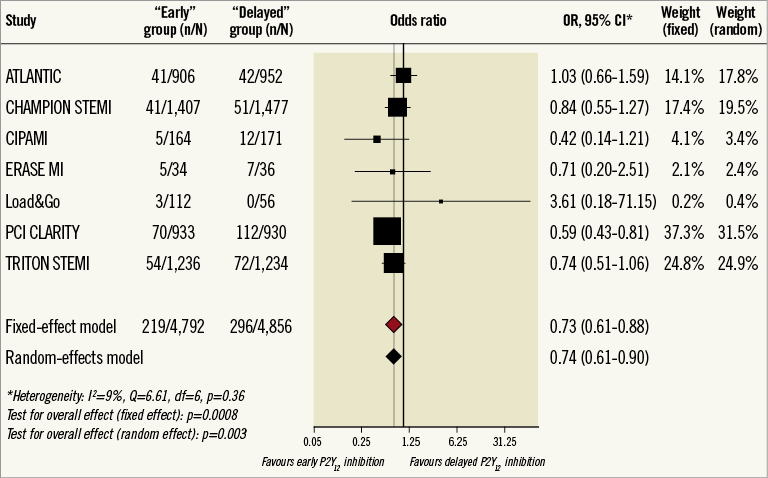
Figure 1. Forest plot for MACE by study. Fixed- and random-effects global results are shown. CI: confidence interval; MACE: major adverse cardiac events
Stent thrombosis information (Supplementary Table 2) was available in four of the seven studies4,14,15,17,18, accounting for 61 events for 7,359 patients (23/3,647 in the “early” group and 38/3,712 in the “delayed” P2Y12 inhibition group). “Early” P2Y12 inhibition was associated with a non-significant reduction in stent thrombosis (OR 0.63, 95% CI: 0.38-1·06, p=0.08); there was no significant heterogeneity between studies for this endpoint (Supplementary Figure 3).
There was no difference between the two strategies for major bleeding (OR 0.87, 95% CI: 0.62-1.21, p=0.41) (Figure 2).
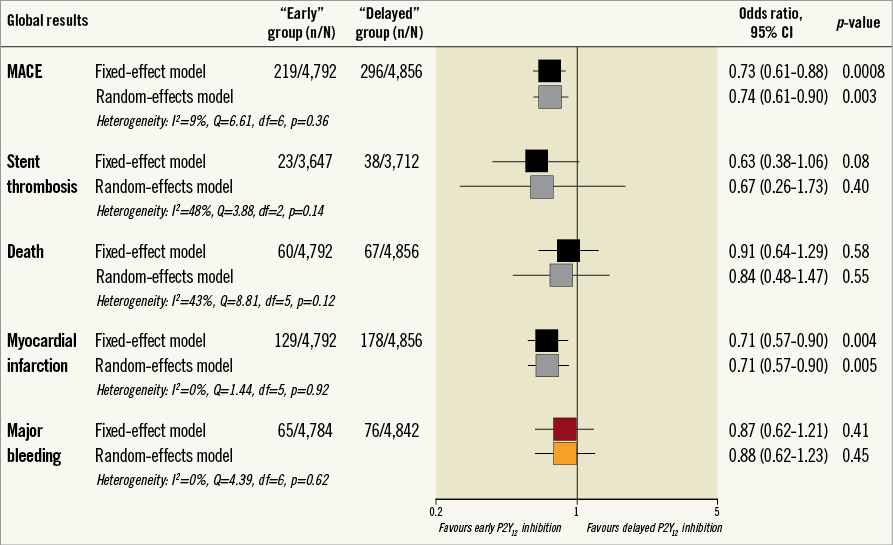
Figure 2. Forest plot with global view on main reported events. Fixed- and random-effects are shown. CI: confidence interval; MACE: major adverse cardiac events
Myocardial infarction (MI) data were available for all studies, with no heterogeneity between studies; “early” P2Y12 inhibition was associated with a significant reduction of MI with both the fixed and random models (Figure 2).
For all-cause death, there was no association between the timing strategy of P2Y12 inhibition and outcomes (Figure 2). This was also the case for CV death, available in four studies3,4,15,17 (28/3,722 in the “early” group vs. 44/3,733 in the “delayed” group; OR 0.63, 95% CI: 0.39-1.01, p=0.05), stroke available in four studies3,4,16,18 (9/3,385 vs. 19/3,379; OR 0.51, 95% CI: 0.24-1.08, p=0.08), and urgent revascularisation available in five studies4,15,16,18,19 (33/3,859 vs. 41/3,926; OR 0.83, 95% CI: 0.52-1.32, p=0.44).
Additional analyses on reperfusion criteria showed a significant impact of early or rapid P2Y12 inhibition on coronary TIMI flow rate before PCI and a reduction of bail-out use of GPI (Supplementary Table 3).
SENSITIVITY ANALYSIS
When comparing the groups of drugs, we found no significant interaction between groups, except for death (Supplementary Table 4).
There was no interaction between the route of P2Y12 inhibitor administration (oral: five studies3,4,17-19, 6,694 patients, vs. IV: two studies14-16, 2,954 patients) and the effect of “early” inhibition on both MACE and stent thrombosis (Supplementary Figure 4, Supplementary Figure 5), as for other endpoints (Supplementary Table 5).
Some interactions were reported (Supplementary Table 6, Supplementary Table 7, Supplementary Figure 4), in particular between the type of strategy (early versus delayed) and the type of PCI (primary versus secondary), but only on death and MACE. In both cases, the effect was mainly driven by the old PCI-CLARITY study (that had a three-day delay between the “early” or “delayed” strategy); the interaction disappeared when it was removed.
ADDITIONAL ANALYSIS WITH PLATO-STEMI DATA (Supplementary Appendix)
Results of the main analysis were not changed for MACE (OR 0.81, 95% CI: 0.71-0.92, p=0.002, I²=22%). The other endpoints are reported in Supplementary Table 8.
The influential analysis did not change the global results except for the old PCI-CLARITY study (Supplementary Table 9).
Discussion
This meta-analysis regrouping nearly 10,000 STEMI patients shows that a strategy of early P2Y12 inhibition before revascularisation 1) is associated with a significant 27% relative risk reduction of MACE (p=0.0008), mainly driven by the 29% relative risk reduction of MI (p=0.004) and, to a lesser degree, a reduction of stent thrombosis (NS); 2) is safe, as it was not associated with an increase of bleeding (it was even associated with a less frequent bail-out use of GPI [p=0.04]); and 3) importantly, is associated with a better coronary reperfusion before stenting (TIMI flow grade 2-3).
The early hours of STEMI are key for the management of patients in terms of delays to reperfusion and to effective P2Y12 inhibition. Recent data suggest a benefit from earlier inhibition in these patients when the diagnosis (STEMI) and the treatment (stenting) are most likely4,18,20. These data are in agreement with older literature concerning the early (pre-hospital) use of GPI in STEMI patients, that showed improved reperfusion and clinical outcomes8,21. However, these studies were all associated with an excess of bleeding complications which is not the case here, with earlier P2Y12 inhibition.
Several important factors interact in the benefit observed and are difficult to interpret individually. One is the time factor, allowing a longer delay to obtain effective P2Y12 inhibition which could explain the apparently more important benefit in secondary PCI. The second factor is the potency and onset of action of the drugs, more favourable with the new oral P2Y12 antagonists than with clopidogrel, which may explain their benefit on MI and stent thrombosis compared with clopidogrel in both TRITON-STEMI and ATLANTIC. The third factor is the IV use of cangrelor with an immediate and potent effect leading to a similar trend on MACE and stent thrombosis, despite a later administration than with prasugrel or ticagrelor. Despite the potential heterogeneity and the difficulties in weighing the roles of these different factors, the results consistently favour early P2Y12 inhibition to prevent ischaemic events with no excess in bleeding risk. This benefit may even be underestimated here for two reasons: 1) although slow gastric absorption and delayed P2Y12 inhibition were likely (STEMI, ±morphine use)18,22 none of the studies used crushed pills, which favour gastric absorption and accelerate the biological efficacy of oral P2Y12 antagonists23,24; 2) none of the studies using cangrelor had the drug administered before or during transfer, something which could confer a greater benefit compared with oral P2Y12 antagonists administered at the time of the procedure.
Interestingly, the benefit of early P2Y12 inhibition in STEMI contrasts with the absence of benefit with early oral P2Y12 inhibition in NSTEMI patients when the diagnosis (NSTEMI) is more difficult to ascertain and the treatment (stenting) is less likely to occur than in STEMI25,26. A similar disconnection between NSTEMI and STEMI was observed for the early use of GPI27.
Limitations
Our meta-analysis is subject to several limitations. We worked on new data obtained from previously published studies to limit the publication bias and to be as exhaustive as possible4,15-17. However, the PLATO STEMI study could not be included because 44% of patients received clopidogrel before randomisation (exclusion criterion), and because we could not obtain the non-published data for the other patients. We provide a supplementary informative analysis including PLATO that does not change our global conclusions. Otherwise, CHAMPION-PCI and CHAMPION-PHOENIX data on STEMI patients were pooled, but differences exist between trials (Supplementary Table 1).
The comparison was carried out between two strategies (early vs. delayed inhibition) using several drugs that differed by their route of administration, onset and intensity of action, and several standards of care. Heterogeneity among studies was searched, fixed- and random-effects models for all the endpoints were provided, and several sensitivity analyses were conducted across groups that consolidated the global result. However, definite conclusions cannot be drawn on the potential influence of a specific medication used and/or a specific study included.
MACE, MI and major bleeding definitions differed across some studies. The duration of follow-up also varied but we were mostly interested in short-term follow-up, when we expect a benefit from a strategy which shortens the time to effective P2Y12 inhibition. Beyond 24 hours after PCI, both strategies had effective P2Y12 inhibition.
Finally, although it is important to reduce MACE with no increase in bleeding rate, our meta-analysis did not show improved survival with this strategy.
Conclusions
A strategy of early effective P2Y12 inhibition significantly reduces MACE and MI in PCI for STEMI, improves coronary reperfusion before PCI, with less frequent bail-out use of GPI without any increase in bleeding complications. We believe that the present meta-analysis supports the current myocardial revascularisation guidelines recommending P2Y12 inhibition before PCI of STEMI7.
| Impact on daily practice As oral drug absorption might be decreased by STEMI patient conditions, additional prospective data are needed to define the optimal delay of pre-treatment with P2Y12 antagonists in this setting. Our meta-analysis found that, in patients with STEMI scheduled to PCI, the early administration of a P2Y12 inhibitor improves coronary reperfusion, reduces MACE, MI and bail-out GPI use without increase in major bleeding, improving the evidence in support of current STEMI guidelines. |
Funding
ACTION Study Group. There was no external source of funding. This meta-analysis was led by the ACTION Study Group (www.action-coeur.org).
Conflict of interest statement
The following authors report receiving research grants or consulting/lecture fees. G. Montalescot: ADIR, Amgen, AstraZeneca, Bayer, Berlin Chimie AG, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, Cardiovascular Research Foundation, Celladon, CME Resources, Daiichi Sankyo, Eli Lilly, Europa, Elsevier, Fédération Française de Cardiologie, Fondazione Anna Maria Sechi per il Cuore, Gilead, ICAN, Janssen, Lead-Up, Menarini, Medtronic, MSD, Pfizer, Sanofi-Aventis, The Medicines Company, TIMI Study Group, WebMD. J-P. Collet: Bristol-Myers Squibb, Sanofi-Aventis, Eli Lilly, Guerbet Medical, Medtronic, Boston Scientific, Cordis, Stago, Centocor, Fondation de France, INSERM, Federation Française de Cardiologie, Société Française de Cardiologie. A. Bellemain-Appaix: Daiichi Sankyo, Eli Lilly, Fédération Française de Cardiologie, Société Française de Cardiologie, AstraZeneca, Servier, Biotronik and Novartis. J. Silvain: Sanofi-Aventis, Daiichi Sankyo, Eli Lilly, Brahms, INSERM, Fédération Française de Cardiologie, Société Française de Cardiologie, AstraZeneca, Iroko Cardio and Servier. M. Cucherat: Bristol-Myers Squibb, AstraZeneca, Bayer, Haute Autorité de Santé. D. Bhatt: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences, Boston VA Research Institute, Society of Cardiovascular Patient Care, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi-Aventis, The Medicines Company, Elsevier, Biotronik, Boston Scientific, St. Jude Medical, FlowCo, PLx Pharma, Takeda. R. Harrington: Amgen, Gilead Sciences, Merck, MyoKardia, TMC, WebMD, AZ, CSL Behring, GSK, Merck, Portola, Regado, Sanofi-Aventis, TMC, Janssen, SignalPath, Scanadu, Element Science. K. Ducci: AstraZeneca. M. Roe: Eli Lilly, Sanofi-Aventis, Daiichi Sankyo, Janssen Pharmaceuticals, Ferring Pharmaceuticals, MyoKardia, AstraZeneca, American College of Cardiology, American Heart Association, Familial Hypercholesterolemia Foundation, PriMed, Boehringer Ingelheim, Merck, Actelion, Amgen, Novartis, Quest Diagnostics, Elsevier Publishers. S. Wiviott: Amgen, Arena, AstraZeneca, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Merck, Sanofi-Aventis, Aegerion, Allergan, Angelmed, Boehringer Ingelheim, Boston Clinical Research Institute, Icon Clinical, Lexicon, St. Jude Medical, Xoma. All conflicts of interest are listed at https://www.dcri.org/about-us/conflict-of-interest. The other authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix. Contributors.
Supplementary Table 1. Included studies.
Supplementary Table 2. Stent thrombosis, major bleeding, MI definitions.
Supplementary Table 3. Additional analyses on the impact of early P2Y12 inhibition on coronary reperfusion criteria.
Supplementary Table 4. Interaction between “early” vs. “delayed” P2Y12 inhibition and clopidogrel vs. ticagrelor/prasugrel vs. cangrelor/elinogrel.
Supplementary Table 5. Interaction between “early” and “delayed” P2Y12 inhibition and route of administration.
Supplementary Table 6. Interaction between “early” vs. “delayed” P2Y12 inhibition and clopidogrel vs. new P2Y12 antagonists.
Supplementary Table 7. Interaction between “early” vs. “delayed” P2Y12 inhibition and primary vs. secondary PCI.
Supplementary Table 8. Additional PLATO STEMI data for the main analysis.
Supplementary Table 9. Sensitivity analysis excluding the PCI-CLARITY data.
Supplementary Figure 1. Study flow diagram.
Supplementary Figure 2. Risk of bias.
Supplementary Figure 3. Forest plot for stent thrombosis.
Supplementary Figure 4. Forest plot for MACE according to STEMI subgroups.
Supplementary Figure 5. Forest plot for stent thrombosis according to STEMI subgroups.
To read the full content of this article, please download the PDF.

