- myocardial infarction
- glycoprotein IIb/IIIa receptor inhibitors
- duration of pre-treatment
- ST-segment deviation
Abstract
Aims: To evaluate the impact of longer duration of pre-hospital initiated antiplatelet and antithrombotic therapy on outcome in patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results: In this sub-analysis of the Ongoing Tirofiban in Myocardial Evaluation (On-TIME) 2 trial, we studied, in 1,370 patients, the effect of pre-treatment time (time from administering study medication to time of angiography) on complete ST-segment resolution (STR), initial patency and 30-day mortality. Study medication consisted of high dose tirofiban (HDT) or control (placebo or no HDT) on top of high dose clopidogrel, aspirin and unfractionated heparin. Median pre-treatment time was 55 min (44-70). Longer pre-treatment was associated with longer transportation times, longer in-hospital delay, longer total ischaemic time (all p<0.001) and higher 30-day mortality (3.6% vs. 1.8%, p=0.046). Longer HDT pre-treatment time was independently associated with increased complete STR both before (odds ratio [OR] 1.51, 95%; confidence interval [CI] 0.98-2.32; p=0.06) and after PCI (OR 1.43, 95%; CI 1.02-2.02; p=0.039) and with a significantly improved initial TIMI 2 or 3 flow (51.4% vs. 43.4%, p=0.042) and reduced 30-day mortality (2.1% vs. 5.0%, p=0.047) as compared to longer control pre-treatment.
Conclusions: Longer time delay before primary PCI is associated with increased mortality. Pre-treatment with high dose tirofiban, however, may compensate for this negative effect by improving ST-segment resolution and initial patency and by reducing mortality. Further studies should be performed to confirm that this is an attractive therapy for patients with longer delays to reperfusion.
Introduction
Time to reperfusion is of utmost importance in patients presenting with ST-segment elevation myocardial infarction (STEMI). Primary percutaneous coronary intervention (PCI) is the preferred therapy provided it can be performed within 90 minutes after first medical contact1. Recently the European Society of Cardiology increased this time window from 90 to 120 minutes and therefore many more patients have become candidates for treatment with primary PCI2. Pre-hospital infarct diagnosis either in the ambulance or at a non-PCI referring centre offers the possibility to initiate pre-treatment with antiplatelet and antithrombotic drugs. So far, guidelines recommend the initiation of aspirin, unfractionated heparin and clopidogrel as soon as possible after first patient contact1,2. The Ongoing Tirofiban in Myocardial Evaluation (On-TIME) 2 study showed that the administration of high dose tirofiban (HDT) on top of these agents is associated with improved ST-segment resolution (STR) and clinical outcome3. With the recent adjustment of the time window to 120 minutes, it is of interest to know whether pre-treatment with triple antiplatelet therapy including HDT is effective in patients with longer delays to PCI as well. Therefore, we evaluated the relationship between the duration of pre-hospital initiated HDT infusion and STR and clinical outcome in the On-TIME 2 trial.
Methods
Patient population
The On-TIME 2 trial enrolled consecutive patients with STEMI who were candidates for primary PCI. In the run-in phase of the On-TIME 2 trial, two intervention centres in The Netherlands enrolled 414 patients in the ambulance to HDT or no HDT in an open-label randomised fashion. This open-label randomised phase was followed by a double-blind, placebo-controlled randomised phase, which enrolled 984 patients in an international multicentre study3. Identical inclusion and exclusion criteria and concomitant treatment regimens were used in both phases from the On-TIME 2 trial, as previously described4,5. Patients were randomly assigned to pre-hospital initiation of HDT (tirofiban [25μg/kg bolus and 0.15μg/kg/min maintenance infusion for 18 h]) or no HDT in the ambulance in addition to 500 mg of aspirin (Aspegic®; Sanofi-Synthélabo France, Le Plessis Robinson, France), 5,000 IU unfractionated heparin administered intravenously and a 600 mg loading dose of clopidogrel. Informed consent was obtained in the ambulance or referring hospital. The study protocol of the On-TIME 2 trial for both the open-label phase and the double-blinded phase was approved by all local ethical committees involved.
In the On-TIME 2 trial all time intervals were recorded in detail. At the time of enrolment of the On-TIME 2 study all participating centres were using femoral access for primary PCI. Total ischaemic time was defined as the time from symptom onset to the time of first balloon inflation during primary PCI. Total ischaemic time consisted of successive time intervals: first, time from symptom onset to STEMI diagnosis by the ambulance employees; second, time from STEMI diagnosis to inclusion, randomisation and administering On-TIME 2 study medication; third, time from initiating study medication to arrival in the hospital; fourth, time from arrival in the hospital to starting angiography; finally, the time from starting angiography to the first balloon inflation. The duration of pre-treatment with HDT infusion in the present analysis was defined as the time interval between starting the study medication to the time of angiography. Door-to-balloon time was defined as time from admission to the hospital until the time of the first inflation of the balloon.
We performed analyses on the extent of ST-deviation resolution before angiography in the PCI centre and at one hour after PCI. Electrocardiograms (ECG) were recorded at three time points: first, pre-hospital at the time of STEMI diagnosis by the ambulance employees; second, before angiography in the cathlab; third, one hour after primary PCI. An independent core lab (Diagram BV, Zwolle, The Netherlands) analysed all ECGs as previously described6,7. Briefly, the sum of ST-segment deviation in all 12 leads was measured 20 minutes after the end of the QRS complex with a calliper. Residual ST-segment deviation was calculated both on the ECG obtained at the catheterisation lab before angiography, and after primary PCI. ST-segment deviation resolution was calculated from paired ECGs and patients were divided into three groups of ST-segment deviation resolution (complete: ≥70% resolution; partial: ≥30% but <70% resolution; and no resolution: <30% resolution), as described previously6,7.
Angiographic assessment was based on the Thrombolysis in Myocardial Infarction (TIMI) flow grade determination of the epicardial infarct related artery (IRA). All angiograms were reviewed by an independent core laboratory (Diagram, Zwolle, The Netherlands), which was blinded to all data apart from the coronary angiograms.
Clinical outcome was assessed at 30 days and at one year. Death was defined as all-cause mortality. Net clinical outcome consisted of the combined incidence of death, recurrent myocardial infarction, urgent target vessel revascularisation, stroke and TIMI major bleeding.
Statistical analysis
Statistical analyses were performed on the combined data from the On-TIME 2 trial double-blind and open label phase8. Baseline characteristics and outcome of the open-label phase and double-blinded phase were analysed for similarity before combining the data. Univariate analyses were conducted with the chi-square test or Fisher’s exact test for categorical variables, the chi-square for trend for ordinal variables, and the Mann-Whitney U test for continuous variables. We performed multivariate logistic regression analyses on the dichotomised resolution score (complete versus partial/no resolution) before and after PCI, with a dummy variable to model the four different combinations of duration of infusion (≤55 minutes or >55 minutes) and randomisation (HDT or control) as the predictor of main interest. We present unadjusted and adjusted models. In the unadjusted model, the dummy variable is the only predictor, with placebo >55 minutes as a reference category. In the adjusted analysis, gender, age (years), current smoking (yes or no) and time from symptom onset to diagnosis (minutes) are included as confounders. Since the adjusted logistic regression analyses have a lower total number due to missing values on some confounders, we performed the unadjusted logistic regression analyses in the group of patients with a valid score on the variables used in the adjusted logistic regression analyses. All p-values were two-sided with significance level p<0.05. The statistical analyses were performed with SPSS for Windows (Rel. 15.0.1.1. 2007; SPSS Inc., Chicago, IL, USA).
Results
Information on the time interval between starting the study medication and angiography was available in 1,370 patients (98.0%) and these patients form the basis of this report. The median pre-treatment time as measured from starting the study medication until the time of the angiography was 55 minutes (IQR 44-70). Baseline characteristics are shown in Table1. Baseline characteristics were comparable, apart from a higher incidence of female and obese patients, in those with longer duration of pre-treatment. Longer pre-treatment was associated with a significantly longer distance to the PCI centre, a longer transportation time, a longer door-to-balloon time and a longer total ischaemic time. Overall and subgroup time intervals according to duration of pre-treatment are shown in Figure1.
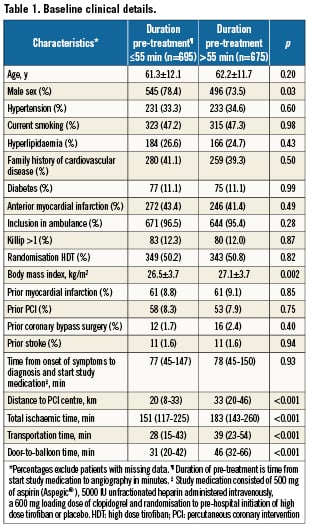
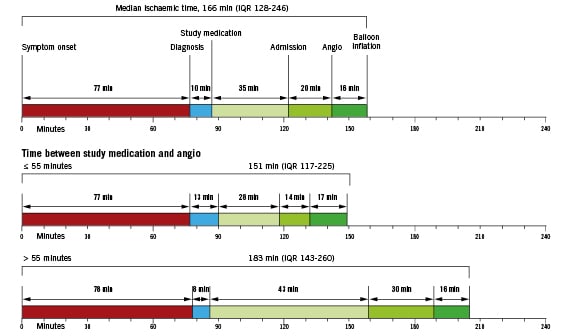
Figure 1. Overall and subgroup time intervals.
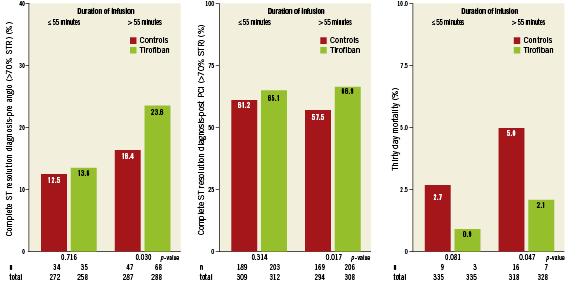
Figure 2. Effect of duration of pre-treatment and randomisation to high dose tirofiban or control on ST-segment resolution and 30-day mortality.
Effect of duration of pre-treatment on ST-segment resolution
Univariate analyses showed that longer pre-treatment duration with HDT was associated with better ST-segment resolution as compared to longer control pre-treatment both before and one hour after PCI, as shown in Figure2. ST resolution before PCI in patients pre-treated for more than 55 minutes was significantly better in patients randomised to HDT as compared to the controls (odds ratio [OR] 1.59, 95%; confidence interval [CI] 1.04-2.42; p=0.031), see unadjusted model in Table2. The same is true for STR one hour after PCI (OR 1.47, 95%; CI 1.05-2.06; p=0.024). In the adjusted model, STR before PCI tended to be better for patients pre-treated with HDT for more than 55 minutes than for the control patients pre-treated for more than 55 minutes (OR 1.51, 95%; CI 0.98-2.32; p=0.06). In the adjusted model for STR one hour after PCI, longer pre-treatment with HDT gave significantly higher odds on complete STR as compared to the controls (OR 1.43, 95%; CI 1.02-2.02; p=0.039). Longer pre-treatment with HDT (>55 minutes) led to significantly better STR before PCI than shorter pre-treatment with HDT (≤55 minutes) (OR 1.98, 95%; CI 1.24-3.15; p=0.004).
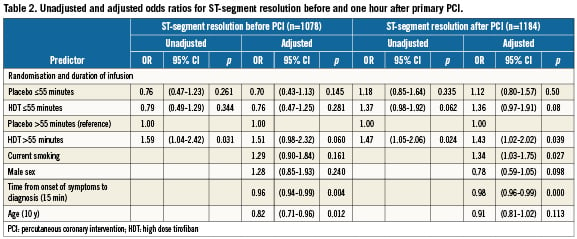
The effect of duration of pre-treatment in tertiles on STR before and one hour after PCI is depicted in Figure3. The p-value for linear trend for complete STR before PCI was 0.08 and 0.07 for HDT pre-treatment and controls, respectively. For complete STR post PCI, the p-value for linear trend was 0.93 and 0.03 for HDT pre-treatment and controls, respectively. This demonstrates a significant decreasing trend to achieve complete STR post PCI in the controls with longer delay to angiography, whereas ST resolution remained high for patients pre-treated with HDT. The number needed to treat with HDT to achieve complete STR in an additional patient before and one hour after primary PCI was 23 and 15, respectively.
Effect of duration of pre-treatment on initial TIMI flow, bleeding, mortality and clinical outcome Initial patency of the IRA as measured by the presence of TIMI 2/3 flow before PCI was not different between HDT or controls with ≤55 minutes pre-treatment (initial TIMI 3 flow 17.7% vs. 16.5%, p=0.70 and initial TIMI 2/3 flow 36.9% vs. 34.3%, p=0.49). However in patients with >55 minutes pre-treatment initial patency was significantly better with HDT as compared to the controls (initial TIMI 3 flow 27.1% vs. 21.8%, p=0.12 and initial TIMI 2/3 flow 51.4% vs. 43.4%, p=0.042).
The incidence of 30-day TIMI major and minor bleeding according to duration of pre-treatment was not different between the HDT group or controls. TIMI major bleeding with ≤55 minutes pre-treatment, 2.4% vs. 1.8%, p=0.59; and with >55 minutes pre-treatment, 4.3% vs. 4.1%, p=0.9. TIMI minor bleeding with ≤55 minutes, 6.0% vs. 4.2%, p = 0.29; and >55 minutes, 6.1% vs. 4.4%, p=0.34. The incidence of TIMI major and minor bleeding in tertiles is depicted in Figure3. The p-value for linear trend for TIMI major and minor bleeding in patients with HDT pre-treatment was not significant (p=0.52 and p=0.84, respectively.).
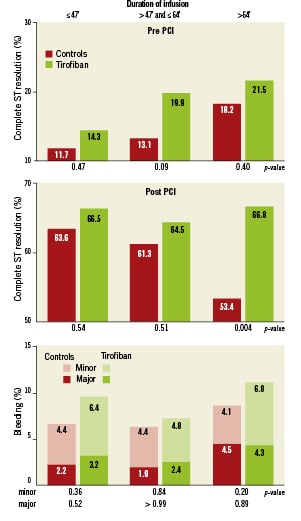
Figure 3. ST-segment resolution before and after primary PCI and TIMI bleeding according to randomisation and duration of pre-treatment in tertiles.
Overall, longer time delay between starting study medication and angiography was associated with a significantly higher 30-day mortality (3.6% [n=23/646] vs. 1.8% [n=12/670], p=0.046). However, HDT pre-treatment during the longer delay was significantly associated with reduced 30-day mortality as compared to the controls (2.1% [n=7/328] vs. 5.0% [n=16/318], p=0.047), see Figure2. Net clinical outcome at 30 days was better in the HDT group as compared to the controls with short (5.1% vs. 9.0%, p=0.049) and longer pre-treatment (9.5% vs. 13.8%, p=0.08).
Discussion
This is the first study that clearly demonstrates that the benefit of pre-treatment with a glycoprotein IIb/IIIa inhibitor is related to the duration of pre-treatment before PCI in patients with STEMI. Patients who were pre-treated with HDT for at least 55 minutes had approximately 1.5-fold higher odds of having complete STR before and after PCI, as compared to pre-treatment with a placebo.
Longer pre-treatment times were associated with longer delay during transportation and in-hospital delay to the cathlab. Not surprisingly, longer transportation time was related to a greater distance to the PCI centre. Figure1 shows that patient delay (time from the onset of symptoms to first medical contact) is not different between the two groups; therefore the difference between the two groups merely reflects extra time delay due to transportation and logistics.
Despite increasing awareness that the time from first medical contact to reperfusion should be as short as possible, delays are still unavoidable due to local geographic conditions. Recently published data from large scale multicentre trials or registries show a median time from diagnosis or first medical contact until balloon inflation varying between 60 and 300 minutes3-7,9. During this time delay, consisting of time to arrange transportation, transportation itself, preparation of the cathlab and time to angiography, a combination of antiplatelet and antithrombotic therapy might be given with the aim to initiate reperfusion and reduce complications during PCI. The international Assessment of the Safety and Efficacy of a New Treatment Strategy with Percutaneous Coronary Intervention (ASSENT-4 PCI) trial showed that full-dose lytic therapy did not improve outcome despite improving initial patency of the infarct related vessel10. Early, pre-hospital initiation of aspirin and unfractionated heparin has been shown to be associated with improved initial patency11. The present substudy from the On-TIME 2 trial showed that a minimum of 55 minutes of pre-treatment with the combination of aspirin, unfractionated heparin, high dose clopidogrel and HDT was associated with a 24% incidence of complete STR before PCI, indicating complete reperfusion in one of every four patients pre-treated with HDT for at least 55 minutes. The data on initial patency confirm the effect of early initiation of HDT to establish reperfusion, especially when time to reperfusion is longer. These results show that glycoprotein IIb/IIIa blockers might exert their beneficial effect already during transportation of the patient to the cathlab and not only during PCI. Previous studies12,13 have demonstrated the very favourable outcome of patients with complete normalisation of ST-segment elevation before PCI and these data were confirmed by our findings: patients with complete STR before PCI had a significantly lower one year mortality (p=0.019) as compared to patients with less than complete STR before PCI. In this analysis we found a significantly lower 30-day mortality in patients with longer pre-treatment with triple antiplatelet therapy as compared to longer pre-treatment with dual antiplatelet therapy. This might be explained by an increased incidence of complete STR both before and after PCI and improved initial patency of the IRV with longer triple antiplatelet pre-treatment.
Recently, other studies also found a beneficial effect of early initiation of a glycoprotein IIb/IIIa blocker. Ortolani and co-workers found that pre-treatment with an IIb/IIIa inhibitor in patients referred to a PCI centre was associated with a better clinical outcome14. The same was found by Hassan et al, where the early administration of abciximab was associated with a 26% incidence of aborted myocardial infarction15. A recent meta-analysis showed improved initial patency and ST resolution in patients pre-treated with a glycoprotein IIb/IIIa blocker16.
The Facilitated Intervention with Enhanced Reperfusion Speed to Stop Events (FINESSE) trial did not find any benefit of facilitation of PCI with abciximab as compared to abciximab given at the time of PCI17. In FINESSE, abciximab was administered relatively late after symptom onset with a median over 160 minutes, whereas in the On-TIME 2 trial study the drug was administered very shortly after symptom onset with a median time of only 77 minutes. The combination of rapid STEMI diagnosis early after symptom onset and immediate pre-hospital initiation of triple antiplatelet therapy allowing longer pre-treatment in case of longer transportation times or longer door-to-balloon times seems to be the key to success in the On-TIME 2 trial.
Limitations
There are some aspects of this analysis that merit careful consideration. First, data from two phases of the On-TIME 2 trial with different design (open-label versus double-blind) were combined. However, both study phases had identical inclusion and exclusion criteria and baseline characteristics, and the outcome of patients included in both study phases were similar4. In addition, an independent corelab and an independent clinical endpoint committee assessed electrocardiographic and angiographic endpoints, as well as clinical endpoints. Second, this analysis was a post hoc analysis and therefore should be viewed as hypothesis-generating and partly explicatory for the positive results of pre-hospital initiation of HDT in STEMI patients on STR one hour post PCI and mortality in the main On-TIME 2 study. Third, the On-TIME 2 study was designed to evaluate the value of early pre-hospital IIb/IIIa blockade on top of dual antiplatelet therapy, including high dose clopidogrel in patients with acute myocardial infarction. Consequently, a limitation of the study is that it does not answer the question of whether initiation of high dose tirofiban other than in the ambulance or referral centre after pre-treatment with high dose clopidogrel is beneficial.
Conclusions
Longer time delay before primary PCI is associated with increased mortality. Pre-treatment with high dose tirofiban may compensate for this negative effect by improving ST-segment resolution and initial patency and therefore reducing mortality. Further studies should be performed to confirm that this is an attractive therapy for patients with longer delays to reperfusion.
Acknowledgement
We thank Vera Derks for her excellent editorial assistance.
Funding
The On-TIME 2 study was partly funded by an unrestricted grant from Merck and Co, Whitehouse Station, NJ, USA.
Conflict of interest statement
A.W.J. van ‘t Hof received speaker fees from Merck, Sanofi Aventis and Schering Plough. C. Hamm received advisory board/speaker fees from Merck, Iroko, Lilly, GlaxoSmithKline, Sanofi Aventis, the Medicines Company, Roche and Abbott. All other authors have no conflict of interest to declare.