Abstract
Aims: The objective of this study was to analyse the outcomes of patients treated with high-bolus dose (HBD) tirofiban compared with abciximab at the time of primary PCI (PPCI) for ST-elevation myocardial infarction (STEMI).
Methods and results: Data from two large UK tertiary centres, with differing protocols for glycoprotein IIb/IIIa inhibitor use during PPCI, were pooled. Propensity scores were calculated based on important covariates, and HBD tirofiban-treated patients were matched to abciximab-treated controls on a one-to-one basis. This resulted in 942 well matched pairs. Survival analysis demonstrated no significant difference in mortality between HBD tirofiban and abciximab either at 30 days (HBD tirofiban 3.7% vs. abciximab 3.2%; HR 1.01 [95% CI: 0.92-1.10], p=0.96) or at three years (HBD tirofiban 9.4% vs. abciximab 9.3%; HR 1.15 [95% CI: 0.79-1.67], p=0.45). Rates of stent thrombosis at 30 days were also similar (HBD tirofiban 12 [1.3%] vs. abciximab 8 [0.8%], p=0.50) but thrombocytopaenia was more common with abciximab (HBD tirofiban 3 [0.3%] vs. abciximab 17 [1.8%], p=0.001).
Conclusions: In this observational study of adjunctive GP IIb/IIIa inhibitor treatment in PPCI, we found no difference in survival between HBD tirofiban-treated patients compared with propensity score-matched abciximab-treated controls up to three-year follow-up.
Introduction
Primary percutaneous coronary intervention (PPCI) has markedly improved outcomes in patients presenting with ST-elevation myocardial infarction (STEMI). However, the optimum strategy for adjunctive pharmacotherapy in PPCI remains an unresolved issue. Several oral and intravenous antiplatelet medications are available, alongside antithrombotic agents such as unfractionated heparin and bivalirudin. In contemporary practice, glycoprotein (GP) IIb/IIIa inhibitors are commonly used in many patients undergoing PPCI1,2. Abciximab has been the standard of care in STEMI, with evidence demonstrating a reduction in death or recurrent myocardial infarction (MI) in this setting3. Tirofiban, a non-peptide antagonist of the GP IIb/IIIa receptor, is used in some centres in a high-bolus dose (HBD) protocol which has recently been approved for use in Europe. We aimed to analyse the real-world outcome in patients treated with HBD tirofiban compared with abciximab in PPCI. We utilised data from two large PPCI units with differing policies on GP IIb/IIIa inhibitor pharmacotherapy in PPCI to facilitate this comparison.
Methods
DATA SOURCES, STUDY POPULATION AND CLINICAL OUTCOMES
The Golden Jubilee National Hospital (GJNH), Glasgow, Scotland, and The James Cook University Hospital (JCUH), Middlesbrough, England, are high-volume PPCI centres providing direct admission and a 24/7 service for their local populations. The GJNH has provided a comprehensive regional PPCI service for Glasgow and south-west Scotland since April 2008 (population 1.6 m). The policy in GJNH is to treat patients presenting with STEMI for PPCI with HBD tirofiban in the cathlab (25 μg/kg bolus and 0.15 μg/kg/min maintenance infusion for 12 hours). The JCUH has performed PPCI since 2003, with a comprehensive service extended to Teesside and County Durham in August 2008 (population 1.4 m). The policy in JCUH has been to administer abciximab in the cathlab (0.25 mg/kg bolus followed by a 0.125 mg/kg/min infusion, to a maximum of 10 mg/min, over 12 hrs) for STEMI patients treated with PPCI. During this study period, both units pre-administered aspirin, and gave weight-adjusted unfractionated heparin as adjunctive therapy. Clopidogrel was pre-administered where possible or was administered immediately after PPCI. Radial access was preferred in both centres.
Data are routinely collected for all PCIs performed and are entered by a combination of clinical and administrative staff according to predefined standardised definitions. The data fields recorded are based on the British Cardiovascular Intervention Society minimum data set, and are therefore readily comparable between centres. Information collected includes demography, comorbidity, clinical presentation and procedural details. Both data sets have data available for mortality through death certification records, providing an accurate and consistent outcome measure. Angiographically confirmed stent thrombosis within 30 days was established by review of repeat procedures. Thrombocytopaenia within seven days of treatment, defined as a platelet count <50×109/L, was identified through laboratory results.
Patients were included in the original study population if they were treated with either HBD tirofiban or abciximab during PPCI for STEMI. Those treated for other indications, including rescue or facilitated PCI, and those not treated with a glycoprotein IIb/IIIa inhibitor were excluded. Only index cases were included. Other major exclusions were use of eptifibatide, no follow-up information available, missing call-to-balloon (CTB) time, and treatment outside the 12-hour PPCI window.
VARIABLES AND DEFINITIONS
Hypertension was a binary variable and defined as blood pressure greater than 140/90 mmHg or treatment with antihypertensive therapy. Hyperlipidaemia was defined as total cholesterol concentration on admission greater than 5.2 mmol/l or prior treatment with a lipid-lowering agent. Diabetes was defined as either type 1 or type 2 diabetes mellitus. Call-to-balloon time, maximum balloon diameter and maximum stented length were analysed as quartiles. Severity of coronary artery disease was defined pre-procedure, and coded as left main stem (LMS)/three-vessel, two-vessel (including the proximal left anterior descending [LAD] artery), two-vessel and single-vessel disease. Multivessel PCI was defined as >1 epicardial vessel territory treated during the index procedure (not including LMS PCI). LMS PCI during the index procedure was coded separately as a binary variable.
STATISTICAL ANALYSIS
Propensity score (PS) matching was used to provide a meaningful comparison between the treatment groups. Among the patients who fulfilled study criteria, many baseline clinical and demographic characteristics varied significantly between HBD tirofiban and abciximab-treated patients (Table 1). Propensity scores (the conditional probability)4 for receiving HBD tirofiban rather than abciximab were calculated for each patient using a logistic regression (LR) model, with treatment assignment the binary dependent variable. Demographic and clinical covariates were selected a priori for entry into the propensity score model if they were thought to influence outcome (death). Included in the LR model were age, gender, previous MI, previous coronary artery bypass graft (CABG), previous PCI, cardiogenic shock, previous cerebrovascular accident (CVA), diabetes mellitus, renal impairment, hypertension, hyperlipidaemia, smoking status, access site, CTB time, severity of coronary artery disease, multivessel and multi-lesion PCI, LMS PCI, drug-eluting stent (DES) use, target vessel, maximum balloon diameter and maximum stented length. A time-period variable (2003-2008, 2009/10, and 2011/12) was included in the model to ensure a contemporary match. The inclusion of such a variable is designed to avoid bias through advances in treatment over time, and through uneven follow-up during survival analysis. The predictive ability of the LR model was assessed using the c-statistic, and goodness-of-fit using the Hosmer-Lemeshow test.
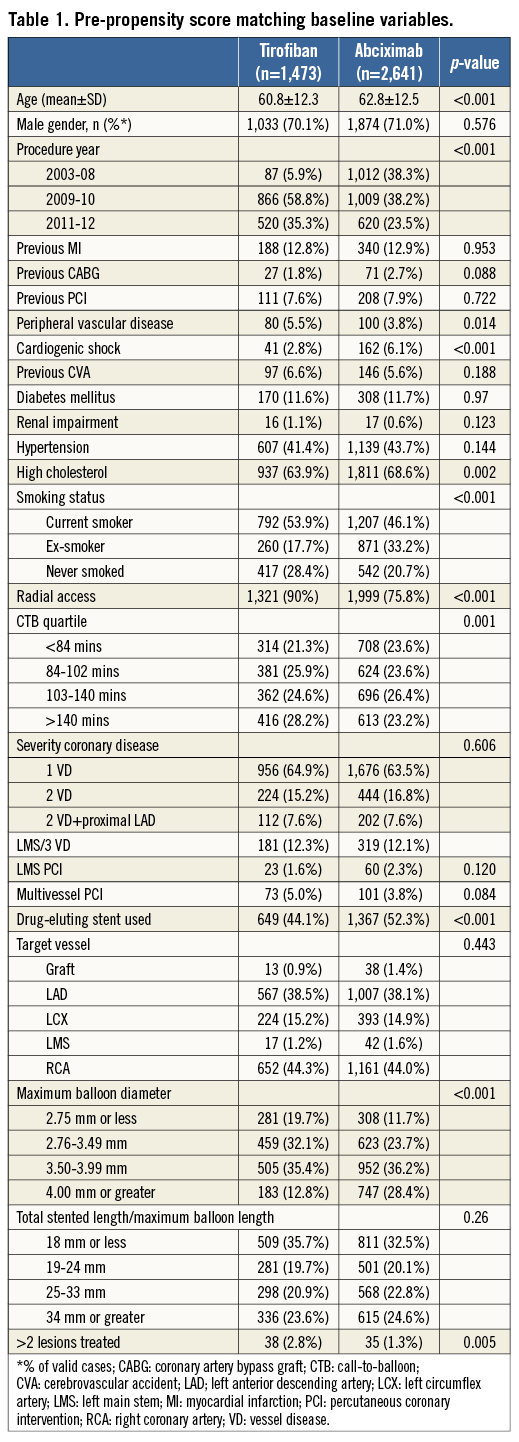
Patients were matched using their individual PS on a one-to-one “nearest neighbour” basis. Abciximab-treated controls were included only once (i.e., matched without replacement). A caliper width of 0.25 standard deviations (SD) was predefined to ensure close matching of HBD tirofiban patients to abciximab-treated controls, and prevent an illogical match. Patients in either group who could not be matched on these criteria were not included in the final analysis. Matched populations were assessed for statistical and standardised differences between the treatment groups.
Categorical data were compared using the chi-square test (unmatched) or McNemar test (matched). Ordinal data were compared using the Mann-Whitney U test (unmatched) or Wilcoxon test (matched). Continuous data were compared using the two-sample t-test (unmatched) or paired two-sample t-test (matched) and expressed as mean (SD). The cumulative probability of outcome-free survival for HBD tirofiban-treated and abciximab-treated patients was determined for death using the Kaplan-Meier product limit estimate from the time of PPCI. Data were censored at the most recently available follow-up or at three years. Probability statistics and hazard ratios comparing outcome for matched HBD tirofiban and abciximab groups were derived from Cox proportional regression analyses stratified by matched pairs to account for the non-independence of groups, with choice of glycoprotein IIb/IIIa the sole predictor variable. A p-value of <0.05 was taken to indicate statistical significance. Exploratory subgroup analysis was performed by gender, age, diabetes mellitus and access site. The matching algorithm was not stratified; therefore, Cox proportional regression survival analyses were adjusted for covariates that were unevenly distributed within the subgroups. Results are presented at 12 months (unadjusted and adjusted). Stent thrombosis at ≤30 days, death or stent thrombosis at ≤30 days and thrombocytopaenia within seven days of the index procedure are reported. PS analysis was carried out using STATA version 9.2 (StataCorp, College Station, TX, USA) while other analyses were performed using IBM SPSS Statistics for Windows, Version 19.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 1,473 STEMI patients were treated with HBD tirofiban during PPCI and there was a pool of 2,641 abciximab-treated controls. Figure 1 outlines the flow of patients through the study. Of all patients presenting with STEMI, the majority (4,504, 89%) received a GP IIb/IIIa inhibitor. Baseline covariates were compared and some clinically important statistical differences were noted between the pre-PS matched groups (Table 1). Following PS matching, 942 pairs of patients (1,884 patients) were included in the final analysis. Table 2 demonstrates that PS matching was successful in generating balanced groups with no statistically significant differences in baseline variables. Standardised differences were less than 5% for each baseline variable. The c-statistic for the PS model was 0.82 (0.80-0.83) indicating excellent discrimination, and the Hosmer-Lemeshow test was non-significant (p=0.295). No significant collinearity was noted among covariates.
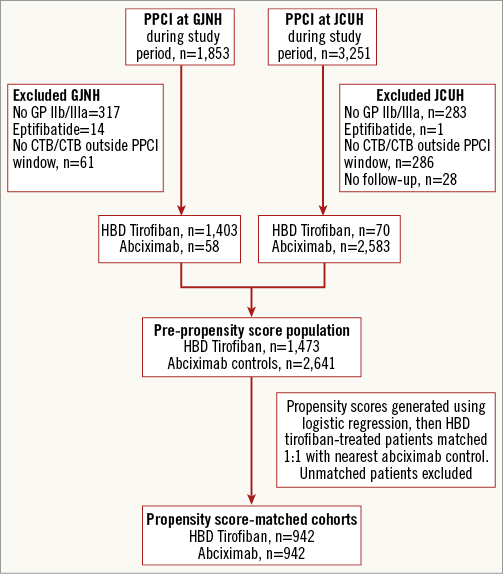
Figure 1. Patient flow. Figure shows number of patients treated at each centre, reasons for patient exclusion and formation of final study cohorts. CTB: call-to-balloon; GJNH: Golden Jubilee National Hospital; HBD Tirofiban: high-bolus dose tirofiban; JCUH: The James Cook University Hospital; PPCI: primary percutaneous coronary intervention
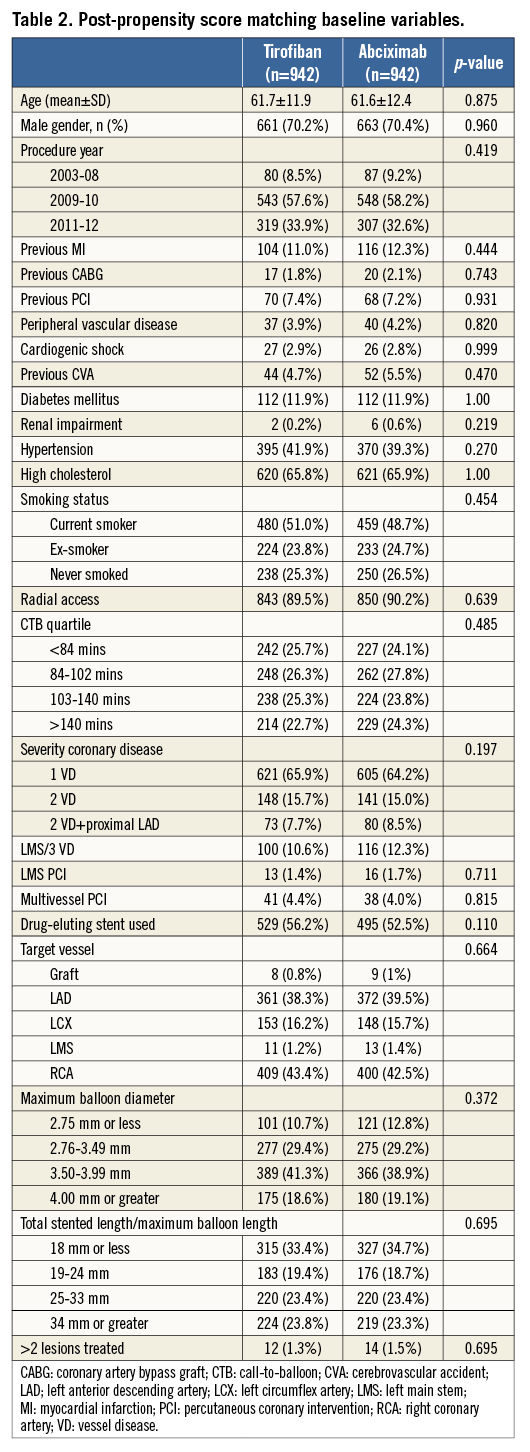
PPCI was performed through the radial route in 1,693 patients (89.9%) and DES were used in 1,024 patients (54.4%). Prior MI was recorded in 220 patients (11.7%) and diabetes mellitus in 224 (11.9%). Multivessel disease was present in around one third of patients (658, 34.9%), although multivessel and/or LMS procedures were less common (114, 5.7%). Median follow-up was 704 days (IQR 336-997 days) and was not different between groups (Wilcoxon test p=0.846). During follow-up there were 74 deaths in each group.
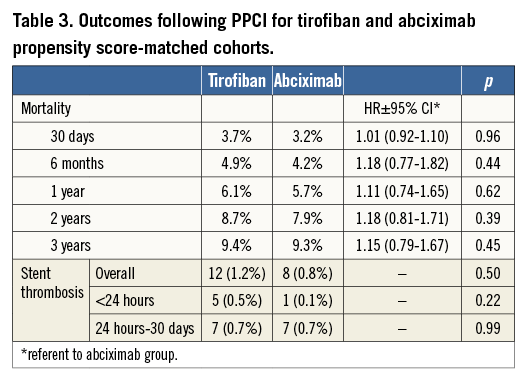
No difference was observed in the rate of death between HBD tirofiban and abciximab-treated cohorts at 30 days following presentation (HBD tirofiban 3.7% versus abciximab 3.2%; hazard ratio [HR] 1.01 [95% CI: 0.92-1.10], p=0.96). Kaplan-Meier survival analysis demonstrated no difference throughout the follow-up period, with a similar pattern of events between the two treatment arms (Figure 2). Table 3 summarises the rates of mortality at time points throughout follow-up. At three years, rates of death were 9.4% for the HBD tirofiban group and 9.3% for the abciximab group; HR 1.15 (95% CI: 0.79-1.67), p=0.45.
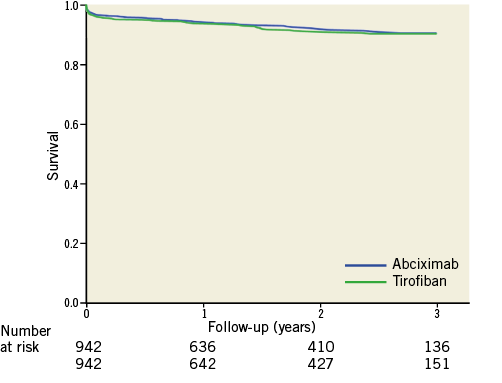
Figure 2. Survival following primary PCI for tirofiban and abciximab propensity score-matched cohorts.
Angiographically confirmed stent thrombosis occurred in six patients within 24 hours and 20 patients at ≤30 days. There was no statistically significant difference between the groups (HBD tirofiban 12 [1.3%] vs. abciximab 8 [0.8%], p=0.50, Table 3). Stent thrombosis or death at ≤30 days was also not different (HBD tirofiban 47 [5.0] vs. abciximab 46 [4.9%], p=1.0).
Thrombocytopaenia following PPCI was recorded in 20 patients and was more common in the abciximab group (HBD tirofiban 3 [0.3%] vs. abciximab 17 [1.8%], p=0.001).
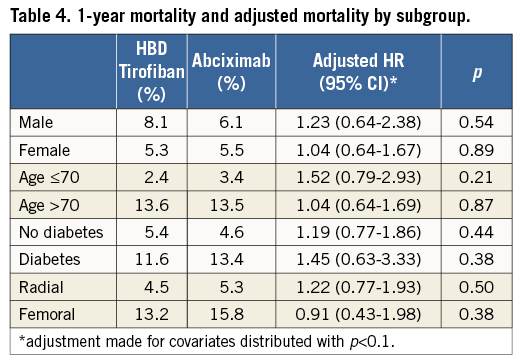
Table 4 summarises subgroup analysis by age, gender, diabetes and access site. Patients over 70 years old, patients with diabetes mellitus and treated via the femoral artery had higher unadjusted mortality, but we found no significant differences in mortality by treatment assignment.
Discussion
At three-year follow-up in this study of adjunctive glycoprotein IIb/IIIa inhibitor treatment in PPCI, we found no difference in survival between HBD tirofiban-treated patients compared with propensity score-matched abciximab controls. We found no difference in acute and subacute stent thrombosis. Significant thrombocytopaenia was more common in the abciximab-treated patients. Patients in this study were treated via the radial access route in the majority of cases (89.9%). The results of this analysis should be seen in the context of the existing evidence on tirofiban, and an evolving evidence base in PPCI including other pharmacotherapy and the importance of access site.
EXISTING CLINICAL EVIDENCE FOR TIROFIBAN
Tirofiban has some potential advantages over abciximab including shorter half-life, lower rates of thrombocytopaenia and lower cost. Tirofiban has been studied in multiple randomised controlled trials (RCTs) in various clinical settings; however, interpretation of the trial results is challenging owing to the different dosing regimens employed. The TARGET study concluded that, in the context of PCI (urgent and elective), tirofiban was associated with an increase in ischaemia-driven endpoints when compared with abciximab (7.6% vs. 6%, HR 1.26, 95% CI: 1.01-1.57, p=0.038)5. This was the largest head-to-head comparison of GP IIb/IIIa inhibitors, but it used the low-bolus dose tirofiban regimen. Subsequent in vivo studies demonstrated inadequate platelet inhibition with this regimen5,6, and further RCTs have studied tirofiban in the HBD formulation. In a comprehensive systematic review and meta-analysis, Valgimigli et al pooled all available randomised data pertaining to tirofiban versus placebo or abciximab. Compared with placebo, HBD tirofiban was associated with a reduction in death (HR 0.68, 95% CI: 0.54-0.86) and combined death/MI (HR 0.69, 95% CI: 0.58-0.81) at 30 days7. When compared with abciximab, the overall results were influenced by the TARGET study. However, in the HBD tirofiban versus abciximab subgroup (n=2,213), no significant difference in death (odds ratio [OR] 0.73, 95% CI: 0.36-1.47) or death/MI (OR 0.87, 95% CI: 0.56-1.35) was observed7.
In the context of PPCI, HBD tirofiban has been studied versus placebo in the On-TIME 2 study8,9. Patients were treated at first medical contact with HBD tirofiban or placebo (n=1,389). Aspirin, high-dose clopidogrel (600 mg) and unfractionated heparin were co-administered to all patients. In the combined open-label and blinded analysis, the primary endpoint of death, reinfarction and urgent TVR within 30 days was lower in the treatment arm (placebo 8.6% vs. tirofiban 5.8%; OR 0.65, 95% CI: p=0.043) and all-cause death numerically less common (placebo 4.1% vs. tirofiban 2.2%, p=0.051), with similar findings at one year (placebo 5.8% vs. tirofiban 3.7%; OR 0.63, 95% CI: 0.38-1.06, p=0.08). Major bleeding occurred in 2.9% of the control group and 3.4% of the tirofiban group (p=0.58). In the MULTISTRATEGY study, HBD tirofiban was compared with abciximab in PPCI in a two-by-two factorial design also comparing BMS with DES (sirolimus-eluting stents): 745 patients were randomised and, overall, no significant difference was noted between abciximab and HBD tirofiban for death or MI at three years (12.9% vs. 12.9%, RR 1.00, 95% CI: 0.67-1.49)10. In keeping with our propensity score-matched cohorts, there was no difference in all-cause mortality at three years (7.8% vs. 6.7%, HR 1.17, 97.5% CI: 0.68-2.02, p=0.56)10.
Furthermore, in the meta-analysis of small molecule glycoprotein IIb/IIIa inhibitors (tirofiban or eptifibatide) versus abciximab in PPCI performed by Gurm et al, no difference was noted at 30 days for mortality (small molecules 1.9% versus abciximab 2.3%; OR 0.84, 95% CI: 0.46-1.55, p=0.58) or reinfarction (small molecules 1.3% vs. abciximab 1.2%; OR 1.22, 95% CI: 0.51 to 2.91, p=0.69)11. In their large observational analysis, Akerblom et al also reported eptifibatide to be non-inferior to abciximab in patients undergoing PPCI at one year (death or MI 0.94 [95% CI: 0.82 to 1.09])12.
That the current analysis has shown similar results to the (albeit small) randomised studies addressing this question suggests an internal validity to the findings. Overall event rates were higher in our analysis, reflecting the higher-risk populations which can be included outside the confines of an RCT. Thus, the current propensity score-matched analysis extends both the number and range of patients studied in this setting.
BLEEDING AND ACCESS SITE IN STEMI
Bleeding is a predictor of poor prognosis in acute coronary syndromes13-16. Bivalirudin, a direct thrombin inhibitor, has shown promising results in the context of PPCI17,18. HORIZONS-AMI, a large open-label RCT, randomised patients to bivalirudin with “bail-out” GP IIb/IIIa or heparin and GP IIb/IIIa. Two thirds of bivalirudin patients also received heparin, and the GP IIb/IIIa inhibitors used were predominantly abciximab or eptifibatide. The benefit of bivalirudin could be seen principally in the reduction of significant bleeding versus the heparin/GP IIb/IIIa arm (4.9% vs. 8.3%, p<0.001). It is hypothesised that the reduction in mortality also observed in the bivalirudin arms at 30 days (2.1% vs. 3.1%, p=0.047) and maintained at three years (5.9% vs. 7.7%, p=0.03) was related to this reduction in bleeding. Indeed, in a secondary analysis of this study, major bleeding was associated with a significantly higher mortality (24.6% vs. 5.4%, p<0.0001) at three years19.
The relative importance of access site, adjunctive pharmacotherapy and bleeding complications, and the interactions between them, are less clear. Currently available data are open to differing interpretations. In HORIZONS-AMI, femoral access was employed in 94% of cases17. In the RIVAL trial, bleeding and vascular complications were significantly reduced with radial compared to femoral access20. Subgroup analysis also suggested that, in STEMI, a mortality benefit may exist for patients treated by experienced radial operators. The subsequent RIFLE-STEACS trial showed a reduction in access-site bleeding (6.8% vs. 2.6%, p=0.003) and 30-day cardiac mortality (9.2% vs. 5.2%, p=0.02) with the use of radial versus femoral access in STEMI. Patients were treated predominantly with a heparin/GP IIb/IIIa inhibitor combination as in our study21. In our data set, radial access was employed in 89.9% of patients. Data on in-hospital bleeding was not available for both cohorts; however, robust information was available for the abciximab arm in whom significant bleeding (haemorrhagic stroke, GI bleed, cardiac tamponade, and requirement for red cell or platelet transfusion) was recorded in 2.9% of patients.
More recently, EUROMAX compared bivalirudin monotherapy and heparin in 2,218 patients, with GP IIb/IIIa used in 11.6% and 69.6% of patients, respectively22. A higher proportion of patients was treated using radial access than in previous studies (n=1,012, 47%). Study medications were commenced at first medical contact, and there was an open-label design. Protocol-defined bleeding was less common with bivalirudin. The main components of excess bleeding were access-site haemorrhage, blood transfusion and asymptomatic reductions in haemoglobin. Definite stent thrombosis within 30 days was significantly more common with bivalirudin (1.6% vs. 0.5%, RR 2.89 [1.14-7.29], p=0.02). The use of prasugrel and ticagrelor in almost half of the patients (49.1%) did not mitigate this excess. One explanation is that whilst prasugrel, for example, has a rapid and predictable response in healthy volunteers and stable patients23,24, in the acute setting of STEMI (with greater platelet reactivity and poorer drug absorption) suboptimal platelet inhibition has been described25. Thus, it can be suggested from currently available data that continued use of GP IIb/IIIa in radial access PPCI is both safe and merited to avoid the complication of early stent thrombosis.
LIMITATIONS AND STRENGTHS
By employing PS matching, we aimed explicitly to remove known factors which may introduce bias. To account for the greater number of patients treated with abciximab earlier in the study period a time-period variable was included in the PS. This ensured that patients were matched both on baseline characteristics and on when they were treated. Available follow-up was thus similar between groups, and the influence of temporal changes in practice was therefore minimised.
As with all observational studies, we accept that unobserved confounding factors may influence the outcome. In particular, non-cardiac comorbidity such as malignant disease and dementia are not systematically recorded and may not therefore be evenly distributed between groups. Systematic differences between hospitals may also influence estimates of treatment effect. Nonetheless, the predictive ability of the PS model was excellent, and the groups were well matched for known baseline variables. Furthermore, such studies of routine practice are complementary to RCTs and unrestrained by the limitations of recruitment, allowing results to be generalised beyond specifically studied populations. Our study had mortality data available for comparison but no comprehensive data available on bleeding or reinfarction. However, mortality is the most appropriate outcome measure in epidemiological outcome studies as it is unambiguous and not subject to measurement bias26.
Conclusions
In this study of adjunctive GP IIb/IIIa inhibitor use in STEMI, HBD tirofiban was compared with a contemporary propensity score-matched abciximab cohort. We demonstrated no difference in mortality up to three years of follow-up.
| Impact on daily practice High-bolus dose tirofiban had equivalent mortality and stent thrombosis rates, and lower thrombocytopaenia compared to abciximab in this observational study. Propensity score-matching was employed to reduce known bias and the results were consistent with the only prior randomised trial assessing this question. High-bolus dose tirofiban could be considered as a lower cost alternative to the current standard of care, abciximab, in primary PCI. |
Acknowledgements
Dr Chih Mun for editing assistance.
Funding
Correvio International Sàrl pharmaceuticals provided financial support for statistical work, but were not involved with conception, study design, data analysis or drafting/approval of the manuscript.
Conflict of interest statement
K. Oldroyd has received speakers fees from Correvio International Sàrl pharmaceuticals. The other authors have no conflicts of interest to declare.

