
Abstract
Aims: We sought to compare the effects of intracoronary administration of a fibrinolytic drug (tenecteplase) to those of a glycoprotein IIb/IIIa inhibitor (abciximab) in patients with ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PPCI).
Methods and results: In this pilot trial, 76 patients (59 male) with anterior STEMI were randomised to intracoronary infusion of reduced-dose tenecteplase or abciximab during PPCI. Angiography was repeated at 48 hours to assess corrected TIMI frame count (cTFC) and TIMI myocardial perfusion grade (TMPG). The primary endpoint was infarct size as assessed by cardiac MRI. The abciximab group showed lower cTFC (median 14.1 [IQR 9.4-17.1]) than the tenecteplase group (18.2 [10.0-28.2]) (p=0.02), and the proportion of patients with TMPG grade 2/3 was higher in the abciximab group (90.3% vs. 67.7%; p=0.03). Major cardiac and cerebrovascular event rates did not differ; however, notably, 2/38 patients in the tenecteplase group experienced subacute stent thrombosis. At four months, there were no significant differences in infarct size between the tenecteplase and abciximab groups (17.0 g [9.6-27.5] vs. 21.1 g [11.3-35.0], p=0.33).
Conclusions: Intracoronary administration of tenecteplase did not reduce infarct size compared to abciximab in STEMI patients undergoing PPCI. Tenecteplase exhibited poorer myocardial reperfusion and might be associated with increased subacute stent thrombosis.
Abbreviations
cTFC: corrected Thrombolysis In Myocardial Infarction frame count
CK: creatine kinase
GPI: glycoprotein IIb/IIIa inhibitors
IC: intracoronary
LAD: left anterior descending artery
MRI: magnetic resonance imaging
PPCI: primary percutaneous coronary intervention
STEMI: ST-segment elevation myocardial infarction
TIMI: Thrombolysis In Myocardial Infarction
TMPG: Thrombolysis In Myocardial Infarction perfusion grade
Introduction
Immediate reopening of acutely occluded coronary arteries by primary percutaneous coronary intervention (PPCI) is considered to be the treatment of choice in patients presenting with ST-segment elevation myocardial infarction (STEMI)1. However, myocardial perfusion is not always optimal after restoration of coronary flow with PPCI2. This is in part due to thrombus embolisation and de novo fibrin formation in the microvascular bed, which can lead to plugging of the microvasculature, microvascular dysfunction and, subsequently, myocardial necrosis2. Some controversy remains regarding the optimal adjunctive antithrombotic therapy during PPCI beyond that of anticoagulants and oral antiplatelet drugs. In this setting, abciximab, a glycoprotein IIb/IIIa inhibitor (GPI), has been shown to improve myocardial perfusion and reduce infarct size3. Clinical experience with intracoronary (IC) fibrinolysis during PPCI is limited, with most studies being observational4,5 or having been performed during the pre-stent era6. There are no randomised trials comparing fibrinolytic drugs to GPI administered via the IC route in patients with STEMI. We hypothesised that local administration of a reduced dose of a fibrin-specific fibrinolytic drug (tenecteplase) could be more effective at dissolving coronary thrombi than administration of an additional antiplatelet agent (abciximab) to patients already receiving protocol-based, dual, oral antiplatelet therapy.
Therefore, we conducted a pilot trial to compare the effects of IC administration of a fibrinolytic agent (tenecteplase) to those of a GPI (abciximab) on myocardial perfusion and infarct size in patients with acute anterior STEMI undergoing PPCI.
Methods
PATIENTS
In this phase-III, single-centre, prospective, randomised controlled trial, patients were considered for enrolment if they were within the first 12 hours of an anterior STEMI and were undergoing PPCI. Inclusion criteria comprised: the presence of symptoms <12 hours, ST-segment elevation ≥0.2 mV in ≥2 precordial leads or new-onset left bundle branch block, and angiographic evidence of a flow-limiting significant lesion in the left descending (LAD) coronary artery (Thrombolysis In Myocardial Infarction [TIMI] flow grade 0-2). Exclusion criteria comprised: cardiogenic shock, history of prior myocardial infarction, contraindication to cardiac magnetic resonance imaging (MRI), chronic kidney disease (serum creatinine >1.5 mg/dl), severe active bleeding, pregnancy, contraindication to study drugs, and severe systemic disease with life expectancy <12 months. The study protocol was approved by the local and regional ethics committees, and was authorised by the Spanish Agency for Drugs and Health Products. The trial was registered in Eudra CT (https://www.clinicaltrialsregister.eu, identifier 2010-022725-16). All patients gave written informed consent to participate in the study.
STUDY PROTOCOL
All patients were given aspirin (300 mg), a loading dose of clopidogrel (600 mg) and unfractionated heparin (70 IU/kg intravenous [IV] bolus) before the procedure. Immediately after the diagnostic angiography, eligible patients were assigned to receive either IC tenecteplase or abciximab according to a block-based computer-generated random sequence. After crossing the infarct-related artery with a guidewire, the study medication (tenecteplase or abciximab) was diluted with 20 ml of saline and infused directly through the guiding catheter for three minutes. Patients randomised to tenecteplase received a reduced dose through the IC route of one fifth of the usual systemic dose, adjusted for weight (e.g., 7.5 mg of tenecteplase for a 75 kg patient). Patients randomised to abciximab received a standard dose (0.25 mg/kg) administered via the IC route, followed by an IV infusion of 0.125 mcg/kg/min for 12 hours. In both groups, PPCI was then performed as usual with implantation of coronary stents as needed. Balloon predilation or manual aspiration of the thrombus was allowed at the operator’s discretion.
All patients were scheduled for repeat catheterisation two days after PPCI. Angiographic images in standardised projections were obtained at 30 frames/s to assess the corrected TIMI frame count (cTFC) and the TIMI myocardial perfusion grade (TMPG, grades 0-3), as previously described7,8. Angiographic images were recorded and assessed offline at an external angiographic core laboratory by a single experienced interventional cardiologist blinded to the study medication. An optimal myocardial reperfusion was defined as a TMPG 2/3.
FOLLOW-UP
After PPCI, serial blood analysis was performed to test for the standard biochemical markers including cardiac troponin I and creatine kinase (CK), which were checked every eight hours over two days. Electrocardiograms were recorded 90 minutes after PPCI to assess ST-segment resolution. Management of STEMI was performed as usual, and patients were discharged on aspirin indefinitely and on clopidogrel for at least one year. Doppler echocardiography with contrast agent was performed before discharge (at five to seven days after PPCI), and was repeated six months later. Cardiac MRI was scheduled four months after PPCI at an external radiological centre. The protocol for cardiac MRI is shown in Supplementary Appendix 1. Total LV myocardial mass and infarct size (expressed as weight of infarct mass in grams and percentage of LV mass) were manually assessed by a single experienced radiologist who was blinded to the study medication, as described previously9. Clinical follow-up was scheduled at 30 days, six months and 12 months after PPCI.
ENDPOINTS
The primary endpoint was the final infarct size assessed by cardiac MRI at four months. Secondary endpoints included: the cTFC and the TMPG on catheterisation 48 hours after PPCI; LV function parameters (volumes and ejection fraction) on cardiac MRI; and a composite of 30-day major adverse cardiac and cerebrovascular events (MACCE) including death, reinfarction (new increase in troponin levels plus suggestive clinical symptoms or new ST-segment changes), stroke and urgent target vessel revascularisation. Major bleeding events were defined as those resulting in death, requirement of blood transfusion, a drop in the haemoglobin level of >4 g/dl, intrapericardial effusion resulting in cardiac tamponade, or any intracranial bleeding.
STATISTICAL ANALYSIS
The sample size in our study was arbitrary and was not previously calculated, because of the scarce data available. After a pre-planned interim analysis, a decision was made to stop enrolment after 76 patients had been included, as explained in Supplementary Appendix 2, which also includes a post hoc analysis on the sample size of the study on the basis of the obtained results (Supplementary Table 1). Group proportions were compared using the chi-square or Fisher’s exact test as appropriate. Differences in the primary and secondary endpoints between the treatment groups were analysed using the non-parametric Mann-Whitney U test. The potential influence of several possible confounding factors (age, diabetes, smoking status, time from pain to dilation, time from door to dilation, proximal versus non-proximal lesions in the LAD, Rentrop collaterals, thrombus burden, balloon predilation and thrombus aspiration) on endpoints was studied. Group means were adjusted for these variables and compared with covariance analysis. The difference between treatment groups with regard to optimal myocardial reperfusion (TMPG grade 2/3 vs. grade 0/1) was first analysed using the chi-square test. Next, a logistic regression model that included the covariates was built. Subgroup analyses were performed, and for this purpose age and time variables were derived as categorical variables. P-values for interaction were acquired using a logistic regression chi-square test, with a pint<0.05 indicating a significant interaction. Thirty-day rates of MACCE were estimated using the Kaplan-Meier method, and the comparison between groups was made using the log-rank test. Multivariate linear regression analysis using a stepwise procedure was conducted to identify predictors of infarct size on the four-month cardiac MRI. All statistical tests were performed with SPSS software, Version 19 (IBM Corp., Armonk, NY, USA). A two-tailed value of p<0.05 was considered statistically significant.
Results
Between August 2012 and April 2016, 102 patients with acute anterior STEMI who met the inclusion criteria for this study were screened for enrolment at our institution (Figure 1). Of those, 26 patients were excluded due to the listed criteria. Therefore, 76 patients were randomised to receive either IC tenecteplase (n=38) or IC abciximab (n=38). There were no adverse events during the IC administration of either drug. Predilation of the culprit lesions and manual thrombus aspirations were performed at the operator’s discretion, but ultimately resulted in the procedures being performed at a similar rate in both treatment groups. The PPCI procedure was completed with implantation of coronary stents in all patients, and there were no immediate complications (Supplementary Table 2).
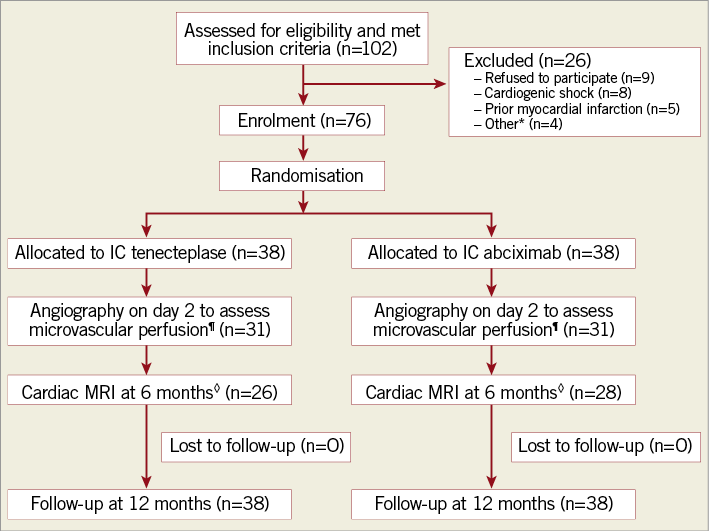
Figure 1. Flow chart demonstrating the trial protocol. *Other reasons for study exclusion were malignant neoplasm (n=2), serum creatinine >1.5 mg/dl (n=1), and advanced liver cirrhosis (n=1). ¶Seven patients in each group refused to undergo second-day angiography. ◊Cardiac MRI was not performed in 12 patients in the IC-tenecteplase group because of claustrophobia (n=5), patient refusal (n=3), death (n=3), and contraindication to cardiac MRI due to defibrillator implantation after the index event (n=1). Cardiac MRI was not performed in 10 patients in the IC-abciximab group because of claustrophobia (n=4), patient refusal (n=2), death (n=3), and technical issues (n=1). IC: intracoronary; MRI: magnetic resonance imaging
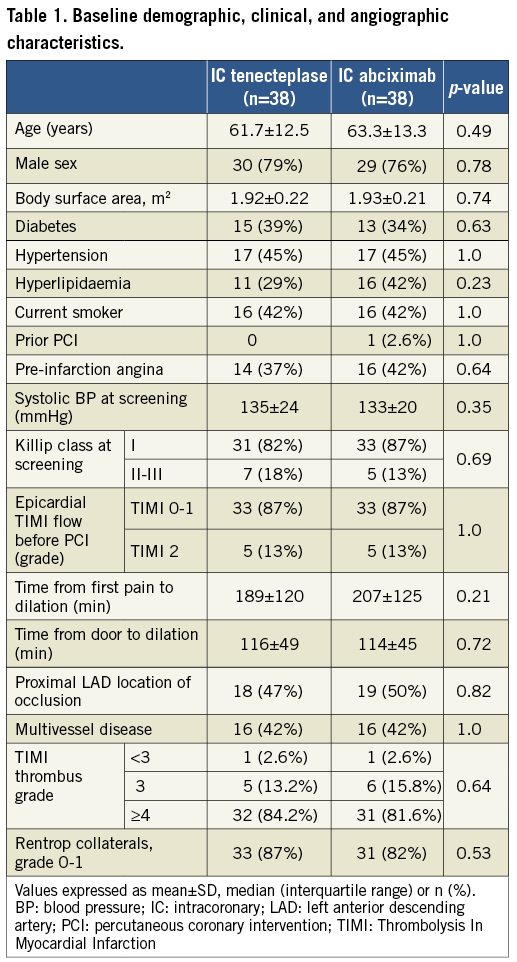
There were no significant differences between the groups with respect to baseline demographic, clinical, angiographic or periprocedural characteristics (Table 1, Supplementary Table 2). All patients continued on aspirin, clopidogrel, angiotensin-converting enzyme inhibitors (except for one patient in the IC-abciximab group because of symptomatic hypotension), beta-blockers and statins throughout the study.
MYOCARDIAL PERFUSION
Microvascular perfusion was significantly better in the IC-abciximab group than in the IC-tenecteplase group at both the immediate and second-day evaluation points (Table 2). On second-day angiography (performed 47.5±19 hours after PPCI), patients in the IC-abciximab group had lower cTFC than patients in the IC-tenecteplase group (median 14.1 [IQR, 9.4 to 17.1] vs. 18.2 [IQR, 10.0 to 28.2], p=0.02) (Figure 2). When adjusted for the previously specified covariates, covariance analysis showed that the significant difference in cTFC between the groups persisted (adjusted mean 14.5±2.0 in IC-abciximab vs. 21.3±2.1 in IC-tenecteplase group, adjusted p=0.03). In multivariate linear regression analysis, the study drug was the only independent predictor of cTFC (p=0.04).
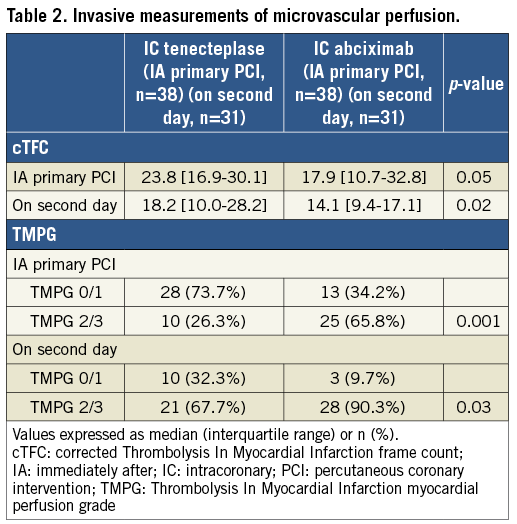
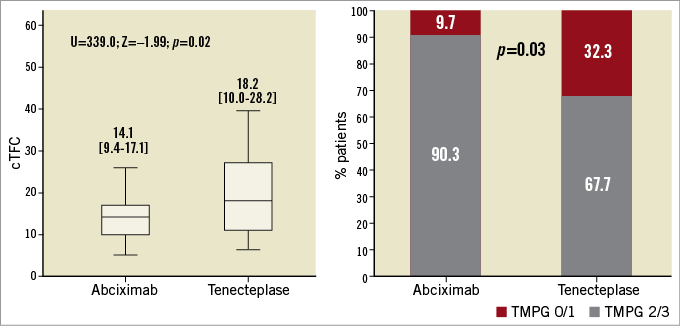
Figure 2. Microvascular perfusion on second-day angiography. A) Differences in cTFC in the abciximab and tenecteplase groups, as assessed by the Mann-Whitney U test. The boxes represent the median values and 25th and 75th (interquartile range) percentiles. B) Percentage of patients in each group with TMPG 2/3 versus TMPG 0/1, as assessed by the chi-square test. cTFC: corrected TIMI frame count; TMPG: Thrombolysis In Myocardial Infarction myocardial perfusion grade
The proportion of patients with optimal myocardial reperfusion (TMPG grade 2/3) on second-day angiography was significantly higher in the IC-abciximab group than in the IC-tenecteplase group (90.3% vs. 67.7%, p=0.03) (Figure 2, Table 2). In multivariate logistic regression analysis, only the study drug abciximab was significantly associated with a TMPG grade 2/3 (p=0.03), while a culprit lesion in the proximal LAD was nearly statistically significant (p=0.05) (Supplementary Table 3). Subgroup analyses were performed to identify potential confounders on TMPG 2/3, but no significant interaction among the covariates and the study medication was observed, except for a borderline interaction for door-to-balloon time <120 min (Figure 3).
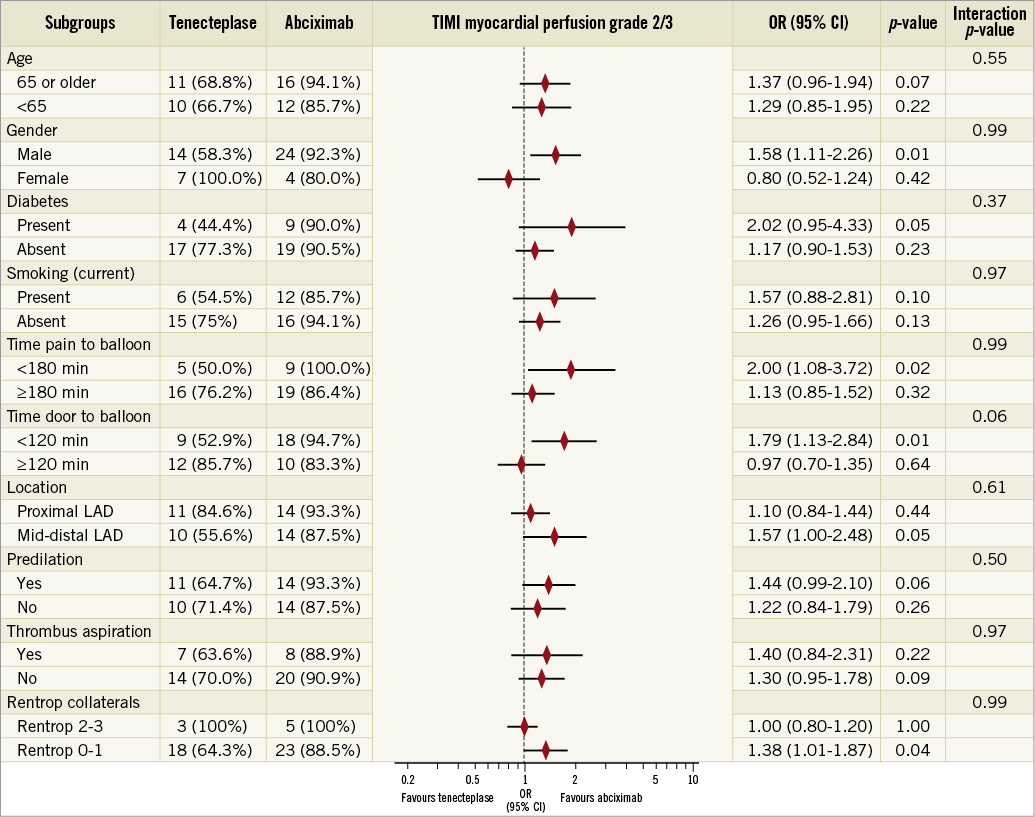
Figure 3. Subgroup analysis on myocardial perfusion. Effect of intracoronary abciximab versus tenecteplase for optimal myocardial reperfusion (TIMI myocardial perfusion grade 2/3) according to different subgroups based on clinical, angiographic, and procedural characteristics. CI: confidence interval; LAD: left anterior descending; OR: odds ratio; TIMI: Thrombolysis In Myocardial Infarction
INFARCT SIZE AND VENTRICULAR FUNCTION
Cardiac MRI was performed at another institution for 52 patients at 4.5±1.6 months. There was no significant difference in infarct size between the IC-tenecteplase and IC-abciximab groups in terms of absolute mass (median 17.0 g [IQR, 9.6-27.5] vs. 21.1 g [IQR, 11.3-35.0], respectively; p=0.33) or percentage of LV mass (15.9% [IQR, 7.5-26.5] vs. 17.0% [IQR, 9.0-25.0], respectively; p=0.59) (Table 3). The results did not change after adjustment for multiple covariates. In multivariate linear regression analysis, absolute infarct size was related to ejection fraction as assessed by echocardiography at five to seven days after PPCI (p=0.02) as well as peak level of CK (p=0.04), whereas infarct size as a percentage of LV mass was only related to peak level of CK (p<0.01) (Supplementary Table 4, Supplementary Table 5).
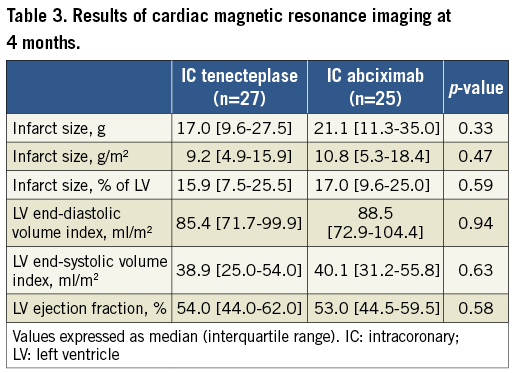
Left ventricular size and systolic function on four-month cardiac MRI did not differ significantly between the study groups (Table 3).
CLINICAL OUTCOMES
During their hospitalisations, two patients (5.3%) in the IC-tenecteplase group died secondary to progressive acute heart failure and cardiogenic shock. Non-fatal subacute definite stent thrombosis occurred in two patients (5.3%) in the IC-tenecteplase group (manifesting clinically as reinfarction in one patient, and subclinically, detected only during the scheduled second-day angiography, in one patient). One diabetic patient in the IC-abciximab group required urgent target vessel revascularisation at 20 days due to significant restenosis presenting as angina and ventricular arrhythmias. Major non-fatal bleeding occurred in one and three patients in the IC-tenecteplase and IC-abciximab groups, respectively. No cases of intracranial haemorrhage were detected; one case of cardiac tamponade occurred in the IC-abciximab group.
Two patients (one in each group) died suddenly within the first three weeks after hospital discharge. There were no significant differences in composite MACCE rates between the groups at the 30-day follow-up (5/38 patients [13.2%] and 2/38 patients [5.3%] in the IC-tenecteplase and IC-abciximab groups, respectively; log-rank test, p=0.22) (Supplementary Figure 1). The global 30-day MACCE and other serious adverse events are summarised in Supplementary Table 6. During longer-term follow-up (up to one year), no additional cardiac deaths occurred, but two non-cardiac deaths (one due to sepsis and another one to neoplasm) were registered in the IC-abciximab group.
Discussion
To our knowledge, this is the first randomised controlled trial comparing the IC administration of a fibrinolytic drug to that of a GPI in patients with STEMI undergoing PPCI. In this pilot and relatively small single-centre study, IC tenecteplase did not significantly reduce the final infarct size compared to abciximab. In addition, it performed worse than abciximab in terms of myocardial perfusion and stent thrombosis.
Several observational studies4,5 and small randomised trials6,10,11 have explored the feasibility of IC thrombolysis. Some researchers have found it to be useful after thrombotic complications during complex PCI4 and during STEMI with massive thrombus and failed aspiration5. In the only reported randomised study of IC fibrinolytics in the era of PPCI and stents, Sezer et al found that streptokinase improved microvascular reperfusion10 and decreased infarct size11 when compared to placebo. Also, some investigators have found IC abciximab administration to be superior to its IV administration for improving myocardial perfusion and reducing infarct size3, but IC abciximab has not been clearly demonstrated to improve clinical outcomes12.
We hypothesised that IC tenecteplase could have a greater potential than abciximab in dissolving thrombus at the epicardial culprit lesion as well as at the microvascular bed. We based our hypothesis on its different mechanism of action, by reducing the fibrin mesh and the established obstructive clots instead of solely blocking the common pathway of platelet aggregation. As there is no approved dose for the IC administration of tenecteplase, we selected one fifth of the systemic dose, which is a similar dose to that given in a previous observational study4. Although our results show a non-significant trend towards smaller infarct size in the IC-tenecteplase group (the primary endpoint of the study), we failed to demonstrate its superiority over abciximab in this relatively small sample size. The fact that the control group did not receive placebo but an active drug with known efficacy in this setting, such as abciximab3, may also have contributed to this negative result.
Our results contrast with the positive findings of Sezer et al with another IC fibrinolytic (streptokinase) after PPCI10,11. There are some differences between that study and ours. First, they compared a reduced dose of IC streptokinase with saline placebo, and all patients received tirofiban in addition to clopidogrel, aspirin and heparin. In contrast, we compared two active drugs (tenecteplase versus abciximab), so patients assigned to IC tenecteplase in our study did not receive any GPI. Another important difference is that they measured infarct size using single-photon emission computed tomography, whilst we used cardiac MRI, which is considered to be the most accurate modality for measuring infarct scars13. Nevertheless, neither their study nor ours found an increased risk of severe bleeding with IC fibrinolytics, with no cases of intracranial haemorrhage or cardiac tamponade reported.
Intracoronary tenecteplase performed worse than abciximab in terms of epicardial flow and myocardial reperfusion, as assessed by cTFC and TMPG. This negative effect was maintained across different subgroups, and it could have some possible explanations. First, we cannot exclude an insufficient antithrombotic effect in the tenecteplase group. This may be due to our empiric election of a relatively low dose of the lytic drug and to the fact that patients in that group did not receive an additional 12-hr perfusion of a parenteral antiplatelet agent. Furthermore, clopidogrel has been progressively displaced by more potent oral antiplatelet drugs such as prasugrel and ticagrelor in the setting of PPCI, although it remains the only P2Y12 inhibitor approved for concomitant use with thrombolytic drugs. Second, clots rich in platelets are more resistant to fibrinolysis, so tenecteplase could have caused the clots to be fragmented rather than dissolved, causing a more extensive embolisation of microthrombi. Third, some authors have reported a prothrombotic effect of fibrinolytic agents, due to the release of thrombin and platelet mediators from the clot mesh when it is only partially lysed, thus reactivating the coagulation cascade and the platelet aggregation process14. This paradoxical effect of IV fibrinolytics has been suggested as a potential explanation for the worsened clinical outcomes after facilitated PCI compared to PPCI for STEMI, including more reinfarctions and culprit vessel closures15. Fourth, fibrinolysis can result in intramyocardial haemorrhage, which could have increased microvascular resistance in the tenecteplase group in our study.
Our results suggest that myocardial reperfusion does not necessarily correlate with final infarct size. This is a controversial issue that has also been observed with other strategies to salvage myocardium in PPCI16. One potential reason is that they reflect different pathophysiological phenomena. Furthermore, they are usually evaluated at different time points after PPCI. Since MRI studies in our patients were scheduled four months after PPCI, oedema and intramyocardial haemorrhage would have resolved at that point and the infarct area may have shrunk.
An unexpected finding in our study was the occurrence of two cases (5.3%) of definite subacute stent thrombosis in the IC-tenecteplase subgroup, as opposed to none in the IC-abciximab group. Although it could be due to chance, it could also be related to the previously mentioned procoagulant side effect of lytic agents. This relatively high rate of stent thrombosis in the tenecteplase group is a matter of concern, and should be further evaluated in several ongoing studies on intracoronary reduced dose of lytic therapy (Supplementary Table 7).
Limitations
Our study has some limitations. This was a relatively small, single-centre pilot trial, so the absence of significant differences in infarct size between the treatment groups may have been related to the small sample size. The trial was single-blind, with the operator knowing the randomisation assignment. However, the angiographic analyses were performed at an external core laboratory and the cardiac MRI images were obtained and analysed at an external radiological centre, in a blinded fashion. Finally, the dose of IC tenecteplase chosen for this study (one fifth of systemic dose) was based on available case reports and observational studies, and we do not rule out that such a dose could have been too low to achieve the desired antithrombotic effect.
This study was designed as a hypothesis-generating study to enable the planning of larger trials based on the results. Once the results of the present study are known, we cannot exclude some potential benefits of IC tenecteplase with regard to infarct size reduction. However, in our opinion, a larger trial of IC fibrinolysis should only be considered if it is administered with concomitant high-intensity antiplatelet therapy (e.g., administering ticagrelor or prasugrel instead of clopidogrel, or infusing GPI by protocol for all patients).
Conclusions
In this pilot trial of patients with acute anterior STEMI undergoing PPCI, IC administration of tenecteplase did not reduce infarct size compared to abciximab. IC tenecteplase showed poorer myocardial reperfusion parameters and a trend towards more subacute stent thrombosis than abciximab, suggesting a potential prothrombotic effect of this fibrinolytic agent, something which deserves further study.
| Impact on daily practice In this first trial comparing adjunctive IC fibrinolytics to GPI during PPCI, we found that administering a reduced dose of IC tenecteplase does not provide any meaningful benefit when compared to abciximab. The combination of a relatively high rate of subacute stent thrombosis and worsened myocardial reperfusion after IC tenecteplase supports the growing suspicion of a prothrombotic effect of fibrinolytics. IC tenecteplase should not be administered without the support of a potent antiplatelet regime. |
Funding
This trial was an investigator-initiated study supported by a research grant from the Ministry of Health of Spain (grant number EC10-257).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Supplementary Appendix 1. Protocol for cardiac MRI.
Supplementary Appendix 2. Sample size: rationale and post hoc analysis.
Supplementary Figure 1. Kaplan-Meier cumulative event curves at 30-day follow-up.
Supplementary Table 1. Results of 4-month cardiac MRI used for post hoc analysis of sample size.
Supplementary Table 2. Periprocedural characteristics of PPCI.
Supplementary Table 3. Logistic regression analysis for optimal myocardial perfusion.
Supplementary Table 4. Multivariate linear regression analysis in relation to infarct size, as absolute mass.
Supplementary Table 5. Multivariate linear regression analysis in relation to infarct size, as a percentage of LV.
Supplementary Table 6. Clinical outcomes and safety data at 30 days.
Supplementary Table 7. Ongoing studies on intracoronary reduced dose of lytic therapy during PPCI.
To read the full content of this article, please download the PDF.

