Coronary no-reflow (CNR) is a frequent phenomenon that develops in patients with ST-segment elevation myocardial infarction (STEMI) after primary percutaneous coronary intervention (PPCI). The incidence of angiographic CNR after PPCI differs widely being reported in between 5%1 and 32%2 of patients by Thrombolysis in Myocardial Infarction (TIMI) grading criteria. The key pathophysiological mechanism of CNR is microvascular obstruction due to the sequential action of myocardial ischaemia, distal embolisation and reperfusion-related injury that may be more frequent in susceptible patients with pre-existing endothelial or microvascular dysfunction (Figure 1). CNR markedly negates the benefits of reperfusion in patients with STEMI and contributes to patients’ poor outcomes, including adverse left ventricular remodelling, new or worsening of heart failure and higher mortality34. So far, no therapy has produced a consistent clinical benefit by preventing or reversing CNR after PPCI. The frequency of CNR differs widely depending on the sensitivity of the diagnostic method and the timing of examination. However, there is no consensus regarding the best method (or timing of examination) that can be used to diagnose CNR after PPCI.
In this issue of EuroIntervention, d’Entremont and colleagues5 assess the effect of thrombectomy on the occurrence of CNR in patients of the TOTAL (Trial Of Routine Aspiration Thrombectomy With Primary PCI Versus Primary PCI Alone in Patients With STEMI) trial. CNR was diagnosed as a TIMI flow grade <3 in the infarct-related artery in the absence of flow-impeding factors at the end of PPCI. CNR was assessed in the angiographic core laboratory (in 1,800 randomly selected patients) and by investigators in the catheterisation laboratory (9,755 patients with available data). CNR was diagnosed in 10.9% of patients (10.7% and 11.1% in the thrombectomy and PCI alone study arms, respectively) in the angiographic core laboratory and in 6.6% of patients in the investigator-based analysis (Cohen’s κ value=0.29 showing a weak interrater agreement). Thrombectomy reduced the frequency of CNR compared with PCI alone in patients who underwent direct stenting (5.1% vs 9.7%) but not in those who did not undergo direct stenting (12.7% vs 10.9%; pint=0.02).
In both analyses, CNR was associated with a higher 1-year incidence of adverse events.
The authors should be congratulated for conducting this study. The study confirms the findings of previous studies regarding the association of CNR with adverse clinical outcomes. Importantly, the study may help to improve the angiographic diagnosis of CNR and suggests that a combination of thrombectomy and direct stenting may reduce the incidence of CNR after PPCI. As the authors emphasise, the study has limitations related to being a post hoc analysis, a non-randomised comparison of direct stenting, as well as having a small sample size for the angiographic core laboratory analysis.
Two findings of this study deserve comment. First, the discrepancy between the angiographic core laboratory and the investigator analyses clearly shows that an investigator-based diagnosis of CNR in the catheterisation laboratory markedly underestimates the true frequency of CNR after PPCI. The finding is somewhat expected considering that the core laboratory staff may have more time and expertise compared with investigators in the catheterisation laboratory – a difference that may be further amplified by differences in the investigators’ expertise in a multicentre study. Although, core laboratory analysis appears to improve the rate of CNR diagnosis, angiographic examination at the end of PPCI procedure markedly underestimates CNR due to the low sensitivity of TIMI grading (TIMI flow grade does not reflect tissue reperfusion) and because it is performed too early in the course of CNR development. Angiographic diagnosis of CNR at the end of PPCI excludes the most important pathophysiological factor of CNR, i.e., reperfusion-related injury, which develops in the hours following blood flow restoration. CNR at the end of PPCI reflects only the contributions of myocardial ischaemia and distal embolisation in the pathophysiology of CNR. Experimental studies of ischaemia/reperfusion in dogs have shown that the area of CNR grows over time, from 9.5% of the initial perfusion defect at 2 minutes to 25.9% at 3.5 hours of reperfusion; notably, areas that had adequate reperfusion at 30 minutes had a marked fall of reperfusion at 3.5 hours6. Other studies in rabbits have shown that regional blood flow may even be hyperaemic at 2 minutes but reduces progressively over 8 hours of reperfusion (most rapidly within the first 2 hours)7. Although, coronary angiography at the end of a PPCI procedure can underestimate CNR, it is highly likely that it will detect the most severe and fixed microvascular obstructions developing over an extensive myocardial area/volume. By finding a higher adjusted risk for all-cause and cardiovascular mortality associated with investigator-based but not core laboratory-based CNR, the study by d’Entremont and colleagues5 appears to support this hypothesis. Second, the thrombectomy-by-direct-stenting interaction, showing that thrombectomy reduced the frequency of CNR only in patients who underwent direct stenting, has potential therapeutic implications but needs cautious interpretation. Since direct stenting was not performed on a randomised basis, it is highly likely that selection bias (direct stenting being more frequent in lower-risk patients with a lower thrombus burden) could have interfered with the outcome. A recent large study including patient-level data from 3 randomised trials showed no thrombus-aspiration-by-direct-stenting interaction with respect to ST-segment resolution or myocardial blush grade8. Thus, only a well-powered 2x2 factorial randomised trial comparing thrombectomy versus no thrombectomy and direct stenting versus no direct stenting could offer a clear answer to this controversy. Notwithstanding the apparent benefits of the thrombectomy/direct stenting combination in the current study, the weak evidence favouring direct stenting and the lack of recommendation to perform thrombectomy during PPCI may not encourage the use of this therapy or the design of a future study. The lower rates of CNR in patients undergoing a combined thrombectomy/direct stenting therapy may point to the role of distal embolisation in the pathophysiology of CNR. Although it may seem counterintuitive, it is highly likely that distal embolisation plays a modest role in the pathophysiology of CNR in STEMI patients. Earlier experimental studies of ischaemia/reperfusion almost unanimously refuted the role of thrombus in CNR and have emphasised that CNR is primarily an ischaemic and reperfusion injury phenomenon. Nearly all studies of thrombectomy and distal protection devices have failed to improve clinical outcome, although they may have improved some markers of reperfusion. Finally, microthrombi detached from culprit lesions during PPCI may be carried into well-reperfused and viable myocardium (directed by the blood stream). One experimental study in dogs showed that embolising particles tend to flow away from the central infarcted area (forced by the already developed CNR) and accumulate in the infarct border leading to infarct expansion9. Thus, distal embolisation may have a modest role in the development of CNR during PPCI.
The prevention and treatment of CNR remains an unmet goal in the therapy of STEMI. The study by d’Entremont and colleagues5 helps to improve the diagnosis of angiographic CNR and may increase interest in seeking therapeutic options that could reduce the occurrence of CNR during PPCI.
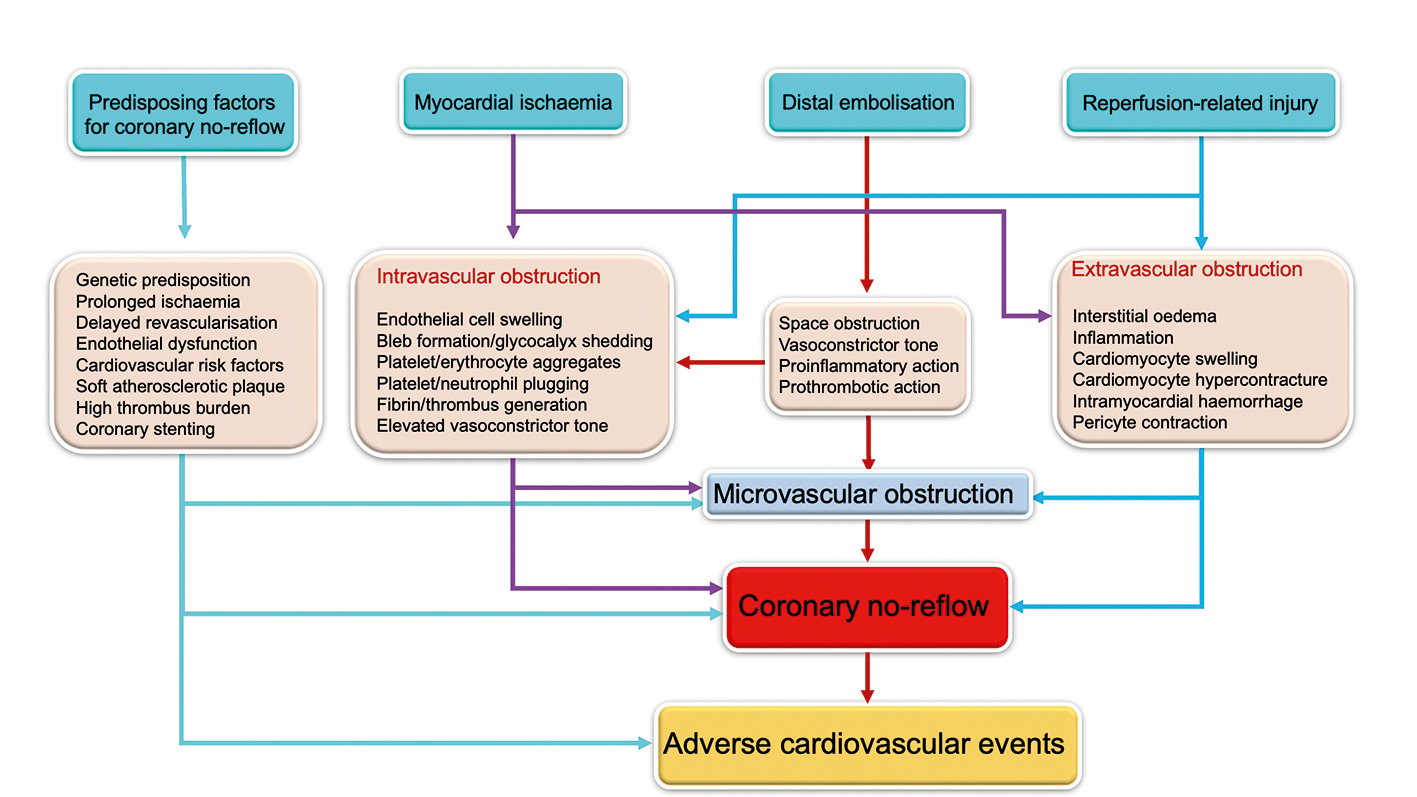
Figure 1. Pathophysiological mechanisms of coronary no-reflow.
Conflict of interest statement
The author has no conflicts of interest to declare.

