
Abstract
Aims: The aim of the study was to evaluate long-term outcomes of transient versus persistent no-reflow.
Methods and results: A total of 17,547 patients with normal flow post percutaneous coronary intervention (PCI) were compared to 590 patients (3.2%) with transient no-reflow and 144 patients (0.8%) with persistent no-reflow. Long-term all-cause mortality was obtained by linkage with the National Death Index (NDI). No-reflow patients were more likely to have presented with ST-elevation myocardial infarction (STEMI), out-of-hospital cardiac arrest (OHCA) or cardiogenic shock (all p<0.01). Long-term NDI-linked all-cause mortality was highest in patients with persistent no-reflow (31%) followed by transient no-reflow (22%) and normal flow (14%) over a median follow-up of 5.2, 5.5 and 4.5 years, respectively (all p<0.0001). Kaplan-Meier survival estimates demonstrated a graded increase in all-cause mortality from normal flow, to transient to persistent no-reflow (p<0.01), with the highest mortality occurring early (<30 days) in the persistent no-reflow group (p<0.0001). Multivariate Cox proportional hazards modelling identified glomerular filtration rate <30 mL/min, ejection fraction <30%, persistent no-reflow and transient no-reflow as independent predictors of increased hazard for all-cause mortality (all p<0.05).
Conclusions: Transient and persistent no-reflow were associated with a stepwise reduction in long-term survival. The presence of even transient no-reflow appears to be an important predictor of adverse long-term outcome.
Abbreviations
ACS: acute coronary syndrome
CABG: coronary artery bypass graft surgery
MIG: Melbourne Interventional Group
NSTEMI: non-ST-elevation myocardial infarction
OHCA: out-of-hospital cardiac arrest
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
Introduction
Angiographic no-reflow is a phenomenon where myocardial blood flow remains impaired despite restoration of epicardial coronary artery patency. It is a serious complication of percutaneous coronary intervention (PCI) with an incidence of 2-30% in various studies1-4.
Predictors of no-reflow have been well documented and include ST-elevation myocardial infarction (STEMI), cardiogenic shock, complex lesions, bifurcation lesions, lesions within prior saphenous vein bypass grafts, poor initial Thrombolysis In Myocardial Infarction (TIMI) flow, chronic kidney disease (CKD)1,5, longer ischaemic time and older age6.
Numerous studies have reported worse short- and long-term outcomes with persistent no-reflow following PCI2,3. However, despite achieving normal angiographic coronary flow at the end of the procedure, we have previously observed that even transient no-reflow during PCI was associated with worse in-hospital and short-term (30-day) outcomes1, a finding supported by several other studies5,7. Few studies have evaluated long-term outcomes of transient no-reflow occurring during PCI. The largest previous study assessing long-term outcomes with transient no-reflow only included 4,329 myocardial infarction patients with a follow-up of three years8.
Given the paucity of data on long-term outcomes with transient no-reflow, we aimed to assess long-term clinical outcomes of transient versus persistent no-reflow.
Methods
PARTICIPANTS
Data from consecutive patients enrolled in the Melbourne Interventional Group (MIG) registry treated with PCI between 2005 and 2013 were analysed.
The MIG registry is a large Australian, multicentre database of consecutive patients treated with PCI at six Australian tertiary hospitals. The MIG registry (http://www.ccretherapeutics.org.au/information/currentresearch/mig.html) has been described in detail previously9,10. Briefly, it records data pertaining to all PCI procedures performed at these centres. Demographic, clinical and procedural characteristics for patients undergoing PCI are prospectively recorded on case report forms using standardised definitions. Follow-up is performed at 30 days and one year. A rigorous auditing process is in place to verify data accuracy11 which approximates 97%, comparable to other large registries12.
The MIG registry is coordinated by the Centre of Cardiovascular Research and Education in Therapeutics, an independent research body within the School of Public Health and Preventive Medicine (Monash University, Australia). The registry was approved by the ethics committee at each participating hospital.
NO-REFLOW DEFINITIONS
Normal antegrade coronary angiographic flow was defined as TIMI grade 3 flow, according to the standard definition developed by the TIMI study group13. Persistent no-reflow was defined as TIMI flow grade ≤2 in the absence of residual coronary stenosis, dissection or spasm, which persisted at the end of the procedure. Transient no-reflow was defined as a temporary reduction in angiographic TIMI flow (TIMI flow grade ≤2), occurring at any time frame during the case which resolved by the completion of the procedure.
CLINICAL OUTCOME DEFINITIONS
In-hospital complications were recorded at the time of hospital discharge or death and medical records were reviewed to verify events. Thirty-day follow-up was conducted by telephone. Events recorded included 30-day and 12-month all-cause mortality, myocardial infarction (MI), target lesion revascularisation (TLR), target vessel revascularisation (TVR), and composite major adverse cardiac events (MACE; consisting of death, MI, and/or TVR). MI was defined as either (1) an increase in creatine kinase or creatine kinase-MB ≥3 times the upper limit of normal and/or (2) significant ST-segment change, development of new Q-waves in ≥2 contiguous electrocardiogram leads, or new left bundle branch block.
Long-term all-cause mortality was obtained by linkage to the Australian National Death Index (NDI), which contains records of all deaths in Australia since 1980. The following variables for each deceased patient were identified: name, date of birth (or estimated year of birth), age at death, gender, date of death, state/territory of registration and registration number. Successful matching of patients was achieved in 99.42% of patients.
Acute renal failure was defined by an increase of serum creatinine to >200 micromol/L (2.27 mg/dL) (or two times the baseline creatinine or new need for dialysis). Stroke was defined by the sudden onset of persistent loss of neurological function caused by an ischaemic or haemorrhagic event during or after PCI. Cardiogenic shock was defined by hypotension (systolic BP <90 mmHg for at least 30 min or needing supportive measures), evidence of end-organ hypoperfusion or cardiac index <2.2 L/(min per m2 and pulmonary capillary wedge pressure of ≥15 mmHg)10.
STATISTICAL ANALYSIS
Continuous variables were expressed as mean±standard deviation, and categorical variables were expressed as numbers/percentages, except where indicated. Continuous variables were compared using the Student’s t-test or Kruskal-Wallis equality-of-populations rank test as appropriate. Categorical variables were compared using Pearson’s chi-square test as appropriate. All calculated p-values were two-sided. P-values <0.05 were considered statistically significant.
The cumulative incidence of all-cause mortality was estimated by the Kaplan-Meier method. The log-rank test was used to evaluate differences between groups.
Cox proportional hazards modelling was used to identify univariate and multivariate predictors of long-term all-cause mortality. Univariate variables with a p-value <0.10 were then included in multivariate models using stepwise backward selection. Variables considered included transient and persistent no-reflow, age, gender, diabetes mellitus, hypertension, dyslipidaemia, renal function (estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73 m2 or 30-59 mL/min/1.73 m2 or <30 mL/min/1.73 m2), previous MI or heart failure (HF), smoking status, peripheral vascular disease, cerebrovascular disease, previous PCI or coronary artery bypass graft surgery (CABG), left ventricular ejection fraction (LVEF) >45% or 30-45% or <30%, acute coronary syndrome (ACS), cardiogenic shock, out-of-hospital cardiac arrest (OHCA), use of intra-aortic balloon pumps (IABP), time from symptom onset to PCI, door to balloon time, treated lesion location, bypass graft lesions, American College of Cardiology and American Heart Association (ACC/AHA) type B2 and C lesions, ostial lesions, bifurcation lesions, chronic total occlusions, use of glycoprotein IIb/IIIa inhibitors, drug-eluting stent use, stent length ≥20 mm, and stent diameter ≤2.5 mm.
All statistical analyses were performed using Stata v14.1 for Windows (StataCorp, College Station, TX, USA).
Results
We analysed data from 18,281 patient procedures (21,605 lesions treated). Of these, 17,547 patients with normal flow were compared against 590 (3.2%) with transient no-reflow and 144 (0.8%) with persistent no-reflow.
The baseline characteristics of the cohorts are presented in Table 1. The incidence of transient or persistent no-reflow was 4% (n=734). Fewer patients with no-reflow compared to patients with normal flow had hypertension, dyslipidaemia or previous PCI or MI (p<0.001 for all). Patients with no-reflow had higher baseline serum creatinine (p=0.02) and lower LVEF (p<0.0001).
Clinical and procedural characteristics are presented in Table 2. Patients with transient or persistent no-reflow were more likely to present with ACS (especially STEMI), cardiogenic shock, OHCA or to be in Killip class IV in the setting of MI. They were more likely to have received IABP support, inotropes, glycoprotein IIb/IIIa inhibitors or have been thrombolysed (all p<0.01). Among patients presenting with STEMI, those with no-reflow had longer door-to-balloon times (p=0.049). Clopidogrel preloading occurred more frequently in those with normal flow (p<0.0001).
Several procedural factors were more prevalent among those with no-reflow including in-stent restenosis, in-stent thrombosis, lesions within bypass grafts, higher-grade stenosis, ACC/AHA type B2/C lesions, chronic total occlusions or pre-PCI TIMI 0 flow (all p<0.01).
No-reflow was associated with higher in-hospital and 30-day all-cause mortality (Figure 1), MACE and heart failure (p<0.0001). The incidence of post-procedural heart failure was 3.4% in the normal flow group, 10.5% in transient no-reflow and 15.3% in persistent no-reflow (p<0.0001). At 12 months, no-reflow was associated with higher all-cause mortality (p<0.0001), cardiovascular mortality (p=0.001), MACE (p<0.0001) and readmissions for heart failure (p=0.012) (Figure 1, Figure 2). Out of all deaths at 12 months (n=829), cardiovascular causes accounted for 58% of deaths among those with normal flow, 71% among those with transient no-reflow and 87% among those with persistent no-reflow (p=0.001).
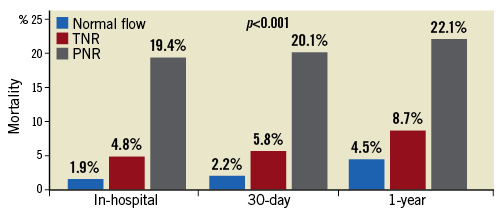
Figure 1. Short- and medium-term all-cause mortality. In-hospital, 30-day and one-year all-cause mortality in patients with normal flow, transient and persistent no-reflow. All-cause mortality was highest in those with persistent no-reflow followed by transient no-reflow and normal flow at all three time points. PNR: persistent no-reflow; TNR: transient no-reflow
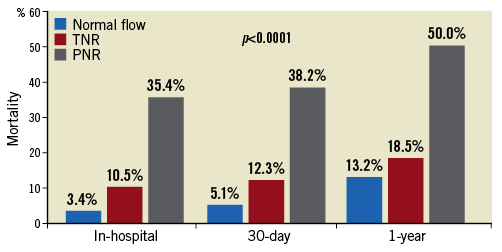
Figure 2. Short- and medium-term MACE. Short-term MACE in patients with normal flow, transient and persistent no-reflow. PNR: persistent no-reflow; TNR: transient no-reflow
No-reflow was associated with higher long-term all-cause mortality over a median follow-up of approximately five years (14% mortality with normal flow, 22% in transient no-reflow [TNR] and 31% in persistent no-reflow [PNR], all p<0.0001) (Figure 3). Kaplan-Meier survival analysis showed a graded increase in all-cause mortality when stratified according to the severity of no-reflow, with the highest mortality occurring early (<30 days) in the persistent no-reflow group (log-rank p<0.0001) (Figure 4).
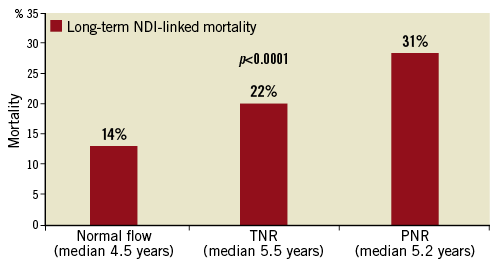
Figure 3. Long-term NDI-linked all-cause mortality. Long-term NDI-linked all-cause mortality in normal flow, transient and persistent no-reflow cohorts over a median of five years.
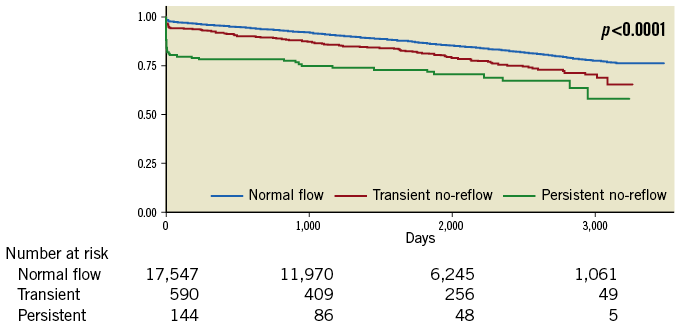
Figure 4. Kaplan-Meier survival estimates. Kaplan-Meier survival estimates of normal flow, transient and persistent no-reflow cohorts.
Landmark analysis in survivors at 30 days showed greater readmissions for heart failure, MACE and major adverse cardiac and cerebrovascular events (MACCE) at 30 days and 12 months in those with no-reflow compared to those with normal flow (all p<0.01). There was higher long-term all-cause NDI-linked mortality in those with no-reflow compared to those with normal flow (12% mortality in those with normal flow, 17% in TNR, 13% in PNR, p<0.001). Kaplan-Meier survival estimates in survivors at 30 days demonstrated highest mortality in those with TNR (Figure 5) compared to the landmark analysis in the first 30 days which showed the highest mortality in the PNR group (Figure 6).
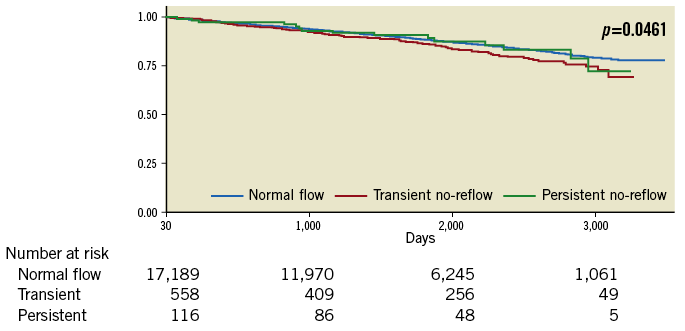
Figure 5. Landmark analysis Kaplan-Meier survival estimates at >30 days. Kaplan-Meier survival estimates in survivors at 30 days demonstrating the highest mortality in those with transient no-reflow.
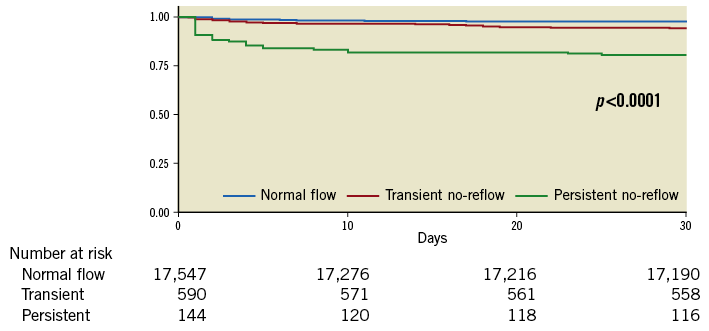
Figure 6. Landmark analysis Kaplan-Meier survival estimates at <30 days. Landmark analysis Kaplan-Meier survival estimates at <30 days showing the greatest mortality in those with persistent no-reflow.
Subgroup analysis excluding patients presenting with cardiogenic shock or OHCA showed greater mortality, MACE and MACCE at 30 days and 12 months in those with no-reflow compared to those with normal flow (all p<0.0001). At twelve months, mortality was 3% in those with normal flow, 5% in TNR, 7% in PNR, MACE was 12% vs. 15% vs. 41% and MACCE was 12% vs. 16% vs. 41%, all p<0.0001. The highest all-cause NDI-linked long-term mortality was seen in TNR (19%) followed by PNR (16%) and normal flow (12%) (all p<0.0001) (Figure 7). Kaplan-Meier survival estimates demonstrated earlier mortality in those with PNR, with greater mortality in the TNR group afterwards (Figure 8).
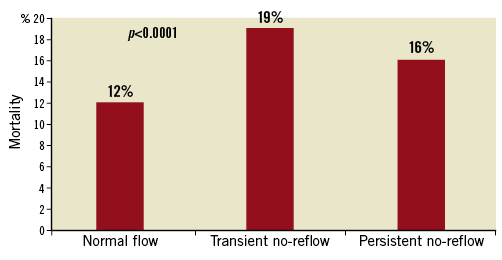
Figure 7. Long-term NDI-linked all-cause mortality (excluding out-of-hospital cardiac arrest and cardiogenic shock). Long-term NDI-linked all-cause mortality (excluding OHCA and cardiogenic shock), demonstrating the greatest mortality in the transient no-reflow group.
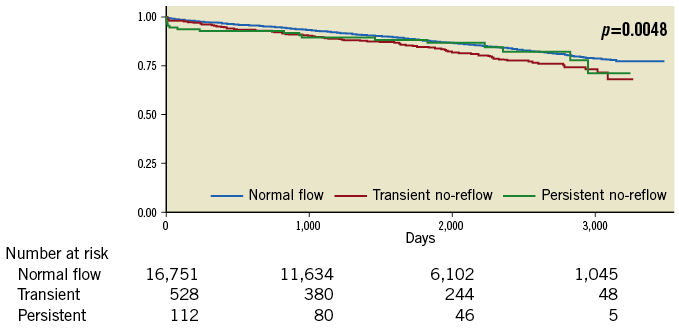
Figure 8. Subgroup analysis Kaplan-Meier survival estimates (excluding cardiogenic shock and out-of-hospital cardiac arrest). Kaplan-Meier survival estimates excluding OHCA and cardiogenic shock, demonstrating greater late mortality in the transient no-reflow group.
Significant multivariate predictors of long-term all-cause mortality included eGFR <30 mL/min/1.73 m2 (HR 3.7, 95% CI: 3.1-4.3), cardiogenic shock (HR 3.2, 95% CI: 2.7-3.8) and LVEF <30% (HR 2.52, 95% CI: 2.1-3) (all p-value <0.001) (Figure 9). While TNR (HR 1.3, 95% CI: 1.1-1.6; p=0.01) and PNR (HR 1.5, 95% CI: 1-2.2; p=0.03) had weaker association with long-term mortality, they remained significant predictors following multivariable adjustment. Both Harrell’s c and Somers’ D estimates (95% CI) suggested very good predictive power for the model with estimates of 79% (78%-80%) and 67% (63%-70%), respectively14.
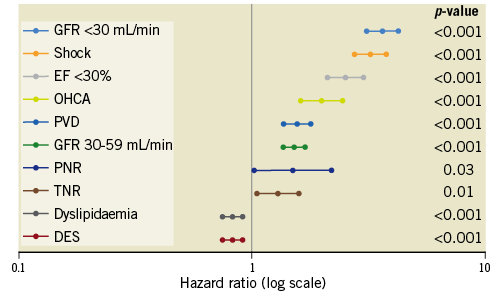
Figure 9. Estimates of hazard ratios and 95% confidence intervals for multivariate predictors of long-term all-cause mortality assessed using Cox proportional hazards modelling. Multivariate predictors of long-term all-cause mortality assessed using Cox proportional hazards modelling. DES: drug-eluting stent; EF: ejection fraction; GFR: glomerular filtration rate; OHCA: out-of-hospital cardiac arrest; PNR: persistent no-reflow; PVD: peripheral vascular disease; TNR: transient no-reflow
Discussion
In this contemporary Australian multicentre registry, representing the largest study assessing the long-term consequences of transient no-reflow, the main finding is that patients with any degree of angiographic no-reflow had worse all-cause mortality at any time point following PCI. Transient and persistent no-reflow were associated with higher long-term all-cause mortality over a median period of approximately five years, underscoring the adverse prognostic value of even transient impairment in myocardial reflow following PCI.
The overall incidence of no-reflow in our study was 4%, with most of these cases representing TNR (3.2%). Our findings of worse in-hospital and short-term mortality with PNR are in accord with other studies1,7,15,16, as is our finding of increased short-term mortality with transient no-reflow7. The higher long-term all-cause mortality in patients with no-reflow is probably due to a multitude of factors. Firstly, patients with no-reflow were more likely to have presented with ACS (especially STEMI), cardiogenic shock, OHCA and to have received IABP and inotropic support. These differences in baseline and procedural characteristics identify a group with very high-risk features at the outset and, not surprisingly, this group had higher short-term mortality, particularly those with persistent no-reflow. However, following multivariable adjustments accounting for these differences, TNR and PNR remained independent predictors of long-term all-cause mortality. While the higher short-term all-cause mortality contributed to the poorer long-term all-cause mortality observed in both no-reflow groups, the Kaplan-Meier analysis (Figure 1) would suggest that cumulative clinical events in the no-reflow groups continued to accrue beyond the 12-month period up to five years. The 30-day landmark and subgroup analyses showed that, both in survivors at 30 days and excluding those presenting with OHCA or cardiogenic shock, the greatest long-term mortality was observed in the TNR group (Figure 5-Figure 8). Several explanations may account for this. Patients with PNR represented a higher risk cohort with the highest short-term mortality (Figure 1, Figure 4). The PNR group was more likely to have associated cardiogenic shock, OHCA, Killip class IV, need for inotropes, complex lesions and TIMI 0 flow pre-PCI (Table 2). Therefore, there were fewer long-term survivors (35% in-hospital mortality in PNR, p<0.0001) (Table 2) and small numbers of patients at risk in this group entered the landmark analysis. Another reason may be that the occurrence of any no-reflow portends poorer clinical outcomes. Whether there is a significant difference in long-term mortality between the TNR and PNR cohorts requires further study and inclusion of a much larger number of patients.
The MIG group has previously examined short-term outcomes of no-reflow1; however, few studies have assessed long-term outcomes of PNR. Ndrepepa et al assessed 1,406 STEMI patients treated with PCI and observed that the five-year mortality was worse in those with no-reflow versus normal flow (18.2% versus 9.5%, OR 2.02, 95% CI: 1.44-2.82, p<0.001)2. Morishima et al in their study of 120 MI patients found that no-reflow was an independent predictor of cardiac death over 5.8 years3. There are fewer studies examining the clinical outcomes of TNR. Recently, Kim et al8 evaluated 4,329 ACS patients in a Korean PCI registry and reported higher all-cause mortality over three years with both TNR and PNR (HR 1.58, 95% CI: 1.11-2.24, p=0.01, and HR 1.98, 95% CI: 1.08-3.65, p=0.028, respectively). The magnitude of the hazard ratios for three-year mortality was largely in keeping with the findings from our study, although our study included stable patients in addition to patients with ACS. Our study, with 18,281 patients, is the largest study underscoring an adverse association between TNR and long-term all-cause mortality up to five years.
There are potential clinical implications arising from our study findings. The adverse short- and long-term mortality associated with TNR and PNR highlights the need for strategies aimed at predicting, preventing and treating no-reflow. Many novel therapies to treat no-reflow have been trialled including direct stenting, thrombectomy devices, distal embolic protection devices, glycoprotein IIb/IIIa inhibitors, adenosine, nicorandil, sodium nitroprusside and calcium channel blockers4,17-20. However, despite promising results in small, phase II studies, no therapeutic agents have to date been shown to impact favourably on long-term outcomes in large, multicentre studies21.
Finally, fewer patients with no-reflow compared to patients with normal flow had hypertension or dyslipidaemia (p<0.0001), prior PCI or MI (p<0.001). Although speculative, this might be due to the use of medical therapies upstream (such as statins, antihypertensives and antiplatelet agents) that might have mitigated against the development of microcirculatory dysfunction and no-reflow22.
Given the worse short- and long-term mortality associated with any no-reflow, these patients may benefit from closer follow-up and more aggressive downstream medical therapy, although such an approach requires further study.
Limitations
Our study findings need to be interpreted with acknowledgement of several limitations. Firstly, although data were collected prospectively, this was a non-randomised, retrospective analysis with differences in the numbers and baseline characteristics across the three groups. Therefore, the results should be considered hypothesis-generating. Secondly, as with any observational study, the possibility of missing data and unmeasured confounders might have influenced the clinical outcomes. Thirdly, while the MIG registry captures a contemporary cohort of patients undergoing PCI, many of the study patients received clopidogrel as opposed to the newer, more potent antiplatelet ticagrelor. Fourthly, the use of agents that might have been used to treat no-reflow was not collected in the MIG registry. Finally, using TIMI flow as a marker of no-reflow might have underestimated the true incidence of impaired tissue perfusion post PCI as patients with normal TIMI flow post PCI may still have impaired tissue perfusion23. However, more objective angiographic measures of no-reflow such as myocardial blush grade and TIMI frame count were not assessed in this registry. Furthermore, the interpretation of TIMI flow grade might have varied amongst cardiologists and core laboratory verification of angiograms was not performed.
Conclusions
Transient and persistent no-reflow following PCI were associated with a stepwise reduction in long-term survival. After adjusting for early mortality and excluding out-of-hospital cardiac arrests and cardiogenic shock, transient no-reflow appeared to be associated with greater long-term mortality. The presence of even transient no-reflow during PCI appears to be an important predictor of adverse long-term outcomes.
| Impact on daily practice Any degree of angiographic no-reflow occurring during PCI, even transient, is associated with worse short- and long-term clinical outcomes. Further randomised controlled studies are needed to assess the effectiveness of preventative and treatment therapies for no-reflow. |
Funding
W. Chan is supported by the National Health and Medical Research Council of Australia (NHMRC) Early Career Fellowship (Neil Hamilton Fairley – Clinical Overseas Fellowship; APP1052960). C.M. Reid is supported by an NHMRC Senior Research Fellowship (APP1045862). S.J. Duffy is supported by an NHMRC grant. M.B. Yudi is supported by a combined NHMRC Postgraduate Scholarship (APP 1115163) and a National Heart Foundation Health Professional Scholarship (Award ID 101130). MIG gratefully acknowledges funding from Abbott, AstraZeneca, Medtronic, MSD, Pfizer, Servier and The Medicines Company. These companies did not have access to data or the right to review manuscripts before publication.
Conflict of interest statement
The authors have no conflicts of interest to declare.

