Abstract
Background: ST-segment elevation myocardial infarction (STEMI) is treated with stenting, but the underlying stenosis is often not severe, and stenting may potentially be omitted.
Aims: The aim of the study was to investigate outcomes of patients with STEMI treated with percutaneous coronary intervention (PCI) without stenting.
Methods: Patients were identified through the DANAMI-3-DEFER study. Stenting was omitted in the patients with stable flow after initial PCI and no significant residual stenosis on the deferral procedure, who were randomised to deferred stenting. These patients were compared to patients randomised to conventional PCI treated with immediate stenting. The primary endpoint was a composite of all-cause mortality, recurrent myocardial infarction (MI), and target vessel revascularisation (TVR).
Results: Of 603 patients randomised to deferred stenting, 84 were treated without stenting, and in patients randomised to conventional PCI (n=612), 590 were treated with immediate stenting. Patients treated with no stenting had a median stenosis of 40%, median vessel diameter of 2.9 mm, and median lesion length of 11.4 mm. During a median follow-up of 3.4 years, the composite endpoint occurred in 14% and 16% in the no and immediate stenting groups, respectively (unadjusted hazard ratio [HR] 0.87, 95% confidence interval [CI]: 0.48-1.60; p=0.66). The association remained non-significant after adjusting for confounders (adjusted HR 0.53, 95% CI: 0.22-1.24; p=0.14). The rates of TVR and recurrent MI were 2% vs 4% (p=0.70) and 4% vs 6% (p=0.43), respectively.
Conclusions: Patients with STEMI, with no significant residual stenosis and stable flow after initial PCI, treated without stenting, had comparable event rates to patients treated with immediate stenting.
Introduction
ST-segment elevation myocardial infarction (STEMI) is mainly due to the rupture of a lipid-rich coronary plaque, resulting in thrombus formation1. The recommended treatment for patients presenting with STEMI is timely primary percutaneous coronary intervention (PCI), including stent implantation2. Coronary stenting and continuous improvement in the composition of the stents available to patients with acute myocardial infarction (MI) have resulted in a dramatic reduction of the incidence of restenosis3. However, stenting per se has not decreased the incidence of cardiac death4, and implantation of a coronary stent is accompanied by various risks of intravascular complications including stent thrombosis and in-stent restenosis56. In STEMI patients, stents may be implanted during primary PCI to restore and secure a normal coronary flow and to prevent re-occlusion and restenosis, but in some patients the coronary flow may be restored and stabilised without stenting. Additionally, the coronary lesions causing STEMI, in general, have a luminal stenosis of less than 75%, and the underlying coronary stenosis per se may not limit coronary blood flow7. Thus, the risk of a plaque rupture with formation of thrombus and subsequent MI may not be determined by the degree of luminal stenosis, but by the plaque content8. That means plaques with only mild non-flow-limiting underlying stenoses, but with lipid-rich arteriosclerotic cores, have been shown to be at high risk of causing future cardiac events9, but stenting these non-flow-limiting lipid-rich plaques does not seem to improve outcome10. Thus, the question remains whether it is safe to omit stenting in STEMI patients with stable and normal coronary flow after initial primary PCI and without a significant underlying stenosis.
Thus, in this post hoc analysis of the Third Danish Study of Optimal Acute Treatment of Patients with STEMI – deferred stenting (DANAMI-3-DEFER), we evaluated the clinical outcome of STEMI patients treated with PCI with no stenting.
Methods
Study design and study population
The DANAMI-3 trial was an open-label, randomised controlled trial evaluating three different revascularisation strategies in patients with STEMI: deferred stenting (DANAMI-3-DEFER)11, ischaemic post-conditioning (DANAMI-3-iPOST)12, and complete revascularisation (DANAMI-3-PRIMULTI)13. Details of the study design, randomisation, treatment allocation, and main results have been published previously1114. The main group of the DANAMI-3-DEFER trial included 1,215 patients randomised 1:1 to either conventional treatment or deferred stent implantation. Furthermore, patients with stenoses in one or more non-infarct-related arteries were also eligible to participate in DANAMI-3-PRIMULTI and were randomised to staged fractional flow reserve-guided complete revascularisation or infarct-related artery-only PCI13.
Patients with STEMI were admitted to one of four primary PCI centres in Denmark, from March 2011 to February 2014, when presenting with ≤12 hours since symptom onset and ST-segment elevation ≥0.1 mV in at least 2 contiguous leads, or documented newly developed left bundle-branch block on electrocardiogram. Exclusion criteria for the main trial were unconsciousness, cardiogenic shock, stent thrombosis, indication for acute coronary artery bypass surgery (CABG), and intolerance to antithrombotic medications, contrast media or anticoagulants.
For the purpose of the present study, patients randomised to deferred stenting in the DANAMI-3-DEFER trial, treated without stent implantation during the index and deferral procedure, were considered as the no stenting group (no stenting). Patients treated with no stenting had ≤30% residual stenosis evaluated visually by angiography, no significant thrombus and/or no visible dissection. The conventional group consisted of patients randomised to conventional PCI and were treated with immediate stenting (immediate stenting). Patients randomised to conventional PCI but treated without stenting for any reason were excluded from the main analysis.
Procedure and outcomes
In the DANAMI-3-DEFER-study, the conventional group was treated with PCI, including stenting and additional antithrombotic medication according to contemporary guidelines. In this group, stenting was strongly recommended and could only be omitted if the operator found stenting contraindicated.
In the deferred stenting group, thrombectomy and balloon dilation was performed to achieve Thrombolysis in Myocardial Infarction (TIMI) flow grade 2 or 3, but the operators were encouraged to use as little mechanical manipulation as possible. After TIMI flow 2 or 3 was established, patients were observed for 10 minutes following coronary guidewire retraction to assure stability of the lesion before sheath removal. Intravenous administration of a glycoprotein IIb/IIIa inhibitor for 12 to 18 hours or bivalirudin for 4 hours post-PCI was encouraged. A repeat coronary angiography with intended stent implantation was performed about 48 hours (but no less than 24 hours) after the index procedure. During the deferral procedure, the culprit lesion was assessed and if deemed stable with ≤30% angiographic residual stenosis by visual assessment, no significant thrombus, and/or no visible dissection, patients were treated without stent implantation. The criterion of ≤30% residual stenosis was based on a pilot study done by our group showing that, in STEMI patients with <30% residual stenosis and no visible thrombus, stenting could safely be omitted, as all lesions in this pilot were patent on a third angiography follow-up15. Irrespective of the degree of residual stenosis, all patients in whom stenting was omitted were offered an optional third angiography at three months. All other procedural interventions and additional treatment were at the discretion of the operator.
The primary endpoint in the present study was a composite of all-cause mortality, recurrent MI, and any unplanned target vessel revascularisation (TVR). Secondary endpoints were the individual components of the primary endpoint, any unplanned target lesion revascularisation (TLR), and hospitalisation for heart failure. All endpoints were identified using the National Danish Heart Registry, local registries of invasive data, and hospital records for validation by an independent clinical events committee. Data between registries were linked via a unique civil registration number16. All Danish residents receive a distinct, personalised, and permanent civil registration number, which enables individual-level linkage between all nationwide health registries unambiguously16.
Coronary angiographic analysis
Angiograms from the deferral coronary angiography following the index procedure were analysed in STEMI patients with no stenting using single-vessel, two-dimensional, catheter calibrated quantitative coronary angiography (Medis Suite 3.2). Minimal lumen diameter, lesion length, reference vessel diameter, proximal and distal lesion diameter, and percentage diameter stenosis were calculated.
Statistical analysis
Categorical variables were compared using the chi-square or Fisher’s exact test and presented as frequencies and percentages. Continuous variables were presented as median and interquartile range (IQR), and the Wilcoxon rank-sum test was used to evaluate differences between groups. Missing values of individual variables are given in Supplementary Table 1. A two-sided p-value ≤0.05 was considered statistically significant in all analyses.
The event rates were calculated for all endpoints, and cumulative incidence curves were calculated for the primary endpoint and the individual components therein. The cumulative incidence curves for recurrent MI and TVR were calculated taking the competing risk of death into account. Gray’s and log-rank tests were used appropriately to compare groups.
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated by both unadjusted and adjusted Cox proportional hazard analysis to show the association between no stenting in patients with STEMI and the endpoints, respectively. To adjust for potential confounders that are known to predict outcomes in patients with STEMI217, the multivariate Cox models were adjusted for multivessel disease, diabetes mellitus, hyperlipidaemia, culprit lesion, use of a glycoprotein IIb/IIIa inhibitor, age, and sex. To avoid overfitting due to a low number of events of TVR, TLR, and hospitalisation for heart failure, this multivariate Cox analysis was adjusted for diabetes mellitus, hyperlipidaemia, multivessel disease, culprit lesion, age, and sex. Both linearity for numeric variables and interaction between any variable included in the adjusted Cox models and the primary endpoint were tested. A test for interaction between no stenting and variables included in the Cox model on the primary endpoint was performed. Plots of the cumulative sum of Martingale-based residuals were used to test the assumption of Cox proportional hazards and found valid18. Additionally, interaction between no stenting and treatment with either complete or culprit-only revascularisation was tested on the primary endpoint, as patients with multivessel disease could be further randomised in DANAMI-3-PRIMULTI.
Supplementary analyses of all patients in DANAMI-3-DEFER, including patients who were revascularised with CABG, were performed, comparing patients treated with: 1) immediate stenting, 2) no stenting, 3) deferred stenting, and 4) randomised to conventional treatment where stenting was omitted. Event rates and cumulative incidence curves were calculated for the primary endpoint comparing all four groups (Supplementary Table 2, Supplementary Figure 1). The log-rank method was used to compare groups. Furthermore, event rates of the primary endpoint were compared between patients treated with no stenting and patients randomised to conventional treatment where stenting was omitted (Supplementary Table 3).
Analyses were performed using SAS (version 9.4, SAS Institute) and R Core Team (2020; The R Foundation)19.
Ethical approval
All participants in the DANAMI-3-DEFER trial provided oral and written informed consent before initiation of any trial-related treatment. The trial was approved by the Central Danish Ethics committee in accordance with the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov: NCT01435408.
Results
Figure 1 shows a flowchart of the study population. A total of 1,215 patients with STEMI were included in DANAMI-3-DEFER, of whom 674 patients were included in the present post hoc study. A total of 84 (13.9%) of the patients randomised to deferred stenting (n=603) did not have a stent implanted (no stenting group) during either the initial procedure or the deferral procedure, and 590 (96.4%) of the patients randomised to conventional PCI (n=612) were treated with immediate stenting during the initial PCI (immediate stenting group).
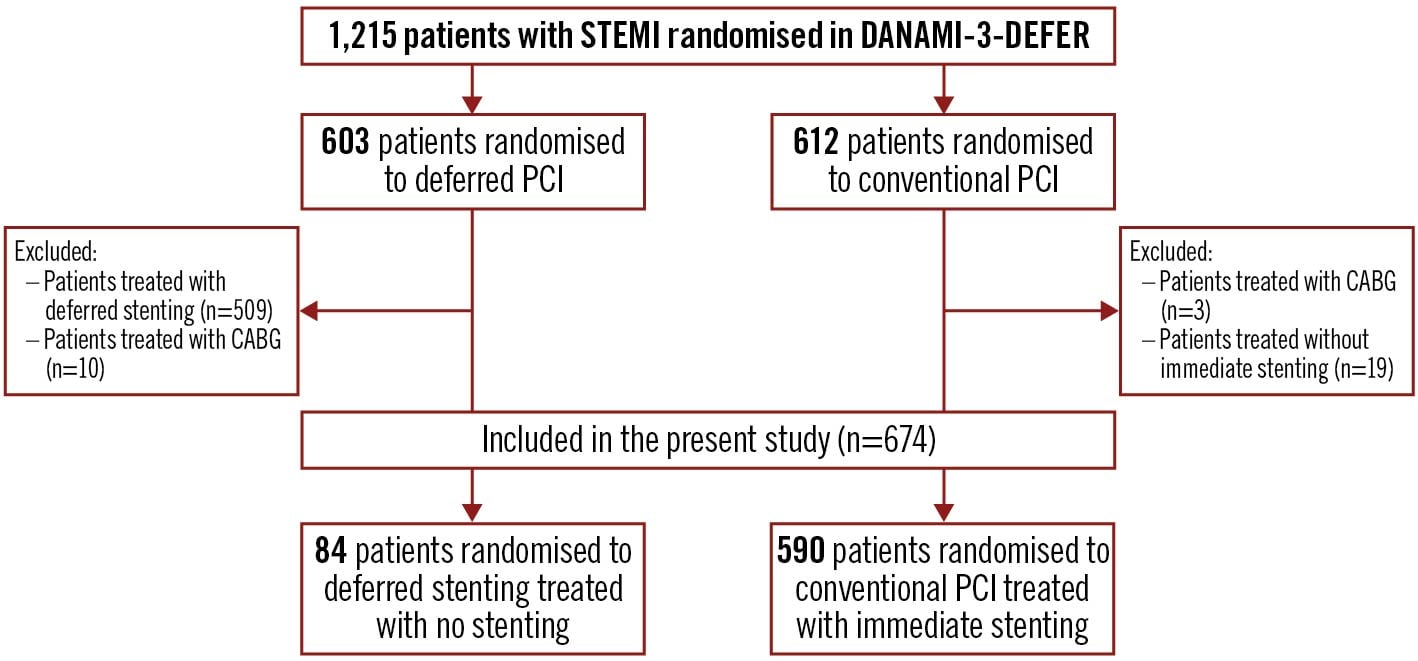
Figure 1. Flowchart of the study population. The selection process of the study population includes both inclusion and exclusion criteria. Arrows indicate the order of the population selection. A total of 674 patients were included, of whom 84 were treated with no stenting and 590 with immediate stenting. CABG: coronary artery bypass graft surgery; DANAMI-3-DEFER: Third DANish Study of Optimal Acute Treatment of Patients With ST-elevation Myocardial Infarction – deferred stenting; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction
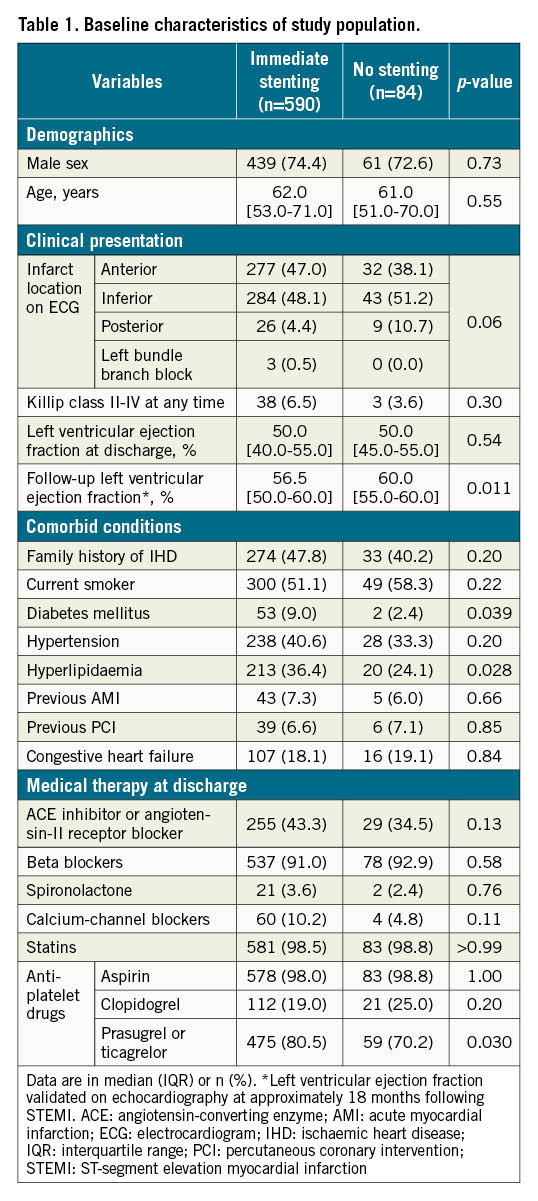
Baseline characteristics including comorbidities and pharmacotherapy at discharge are shown in Table 1. In patients treated without stenting, fewer had diabetes mellitus and hyperlipidaemia and they were less likely to receive prasugrel or ticagrelor at discharge compared to patients treated with immediate stenting.
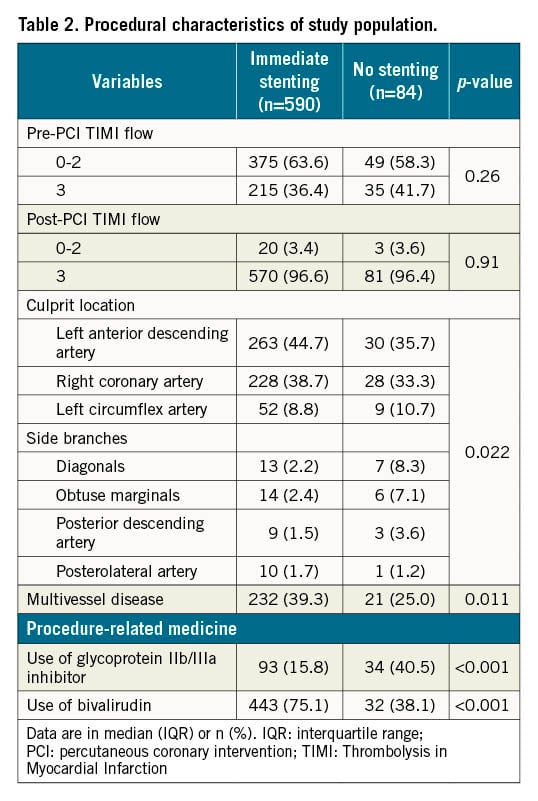
Procedure-related characteristics are presented in Table 2. In patients treated with no stenting, 80% had a culprit lesion of one of the three main coronary arteries: right coronary artery, left circumflex artery, and left anterior descending artery. However, the culprit lesion in patients with no stenting was more frequently in a side branch of a main coronary artery compared to patients treated with immediate stenting. In the immediate stenting group, patients were more likely to have multivessel disease, and less likely to receive a glycoprotein IIb/IIIa inhibitor compared to the no stenting group. Of the 84 patients treated with no stenting, 60 patients (71%) were treated with plain old balloon angioplasty (POBA), 6 patients (7%) with thrombectomy only, and 18 patients (21%) underwent no intervention during the initial procedure (Table 3).
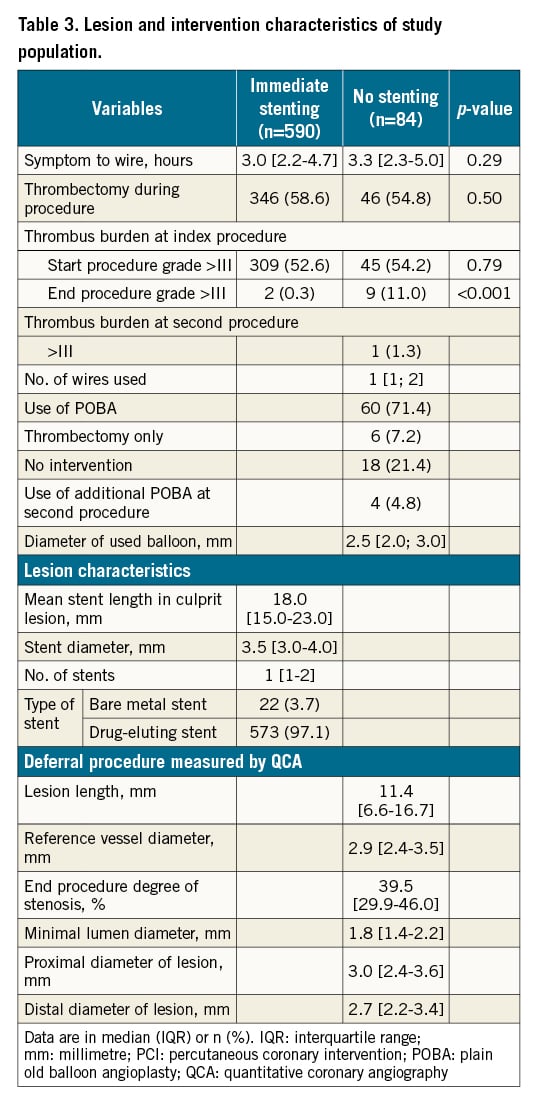
Lesion characteristics are presented in Table 3. At the deferral procedure, patients treated with no stenting had a median degree of stenosis at the end of the procedure of 40%, a median reference vessel diameter of 2.9 mm, and a median lesion length of 11.4 mm measured by quantitative coronary angiography. The majority of patients treated with immediate stenting had a drug-eluting stent implanted. The implanted stents had a median length of 18.0 mm and a median diameter of 3.5 mm. A total of 33 patients treated without stenting were referred for a third angiography at 3 months due to residual stenosis and thrombus. The median residual stenosis in the culprit lesion at the angiography was 30% (IQR 0-50%) and no patients had thrombus. Two patients were treated with stenting at the 3-month follow-up due to significant residual stenosis. These two patients were considered as TLR and were handled as such (Table 4).
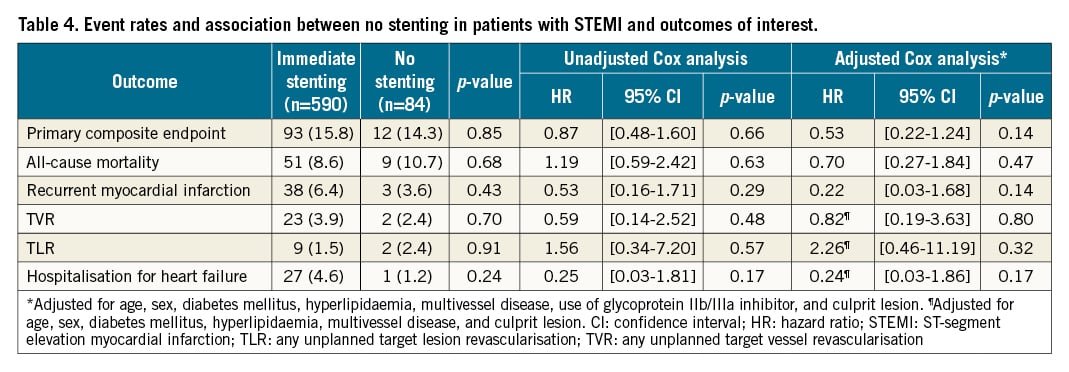
The median follow-up period was 3.4 years (IQR 2.6-4.1). The cumulative incidence curves of the primary endpoint and the individual components are presented in Figure 2. The primary endpoint occurred in 12 patients (14%) who were treated with no stenting and in 93 patients (16%) who were treated with immediate stenting. No stenting was not associated with an increased risk of the primary composite endpoint of all-cause mortality, TVR, and recurrent MI (HR 0.87, 95% CI: 0.48-1.60; p=0.66) (Central illustration). The incidence of TVR was 2.4% and 3.9% (p=0.70) for no stenting versus immediate stenting, and the incidence of recurrent MI was 3.6% and 6.4% (p=0.43) (Table 4). No difference was observed between treatment with no stenting or immediate stenting on any of the individual components of the primary endpoints, TLR, or hospitalisation for heart failure. The association between treatment with no stenting and the primary endpoint remained non-significant in the multivariate Cox analysis (adjusted HR 0.53, 95% CI: 0.22-1.24; p=0.14) (Table 4). There was no interaction between full revascularisation and no stenting on the primary endpoint (p=0.99).
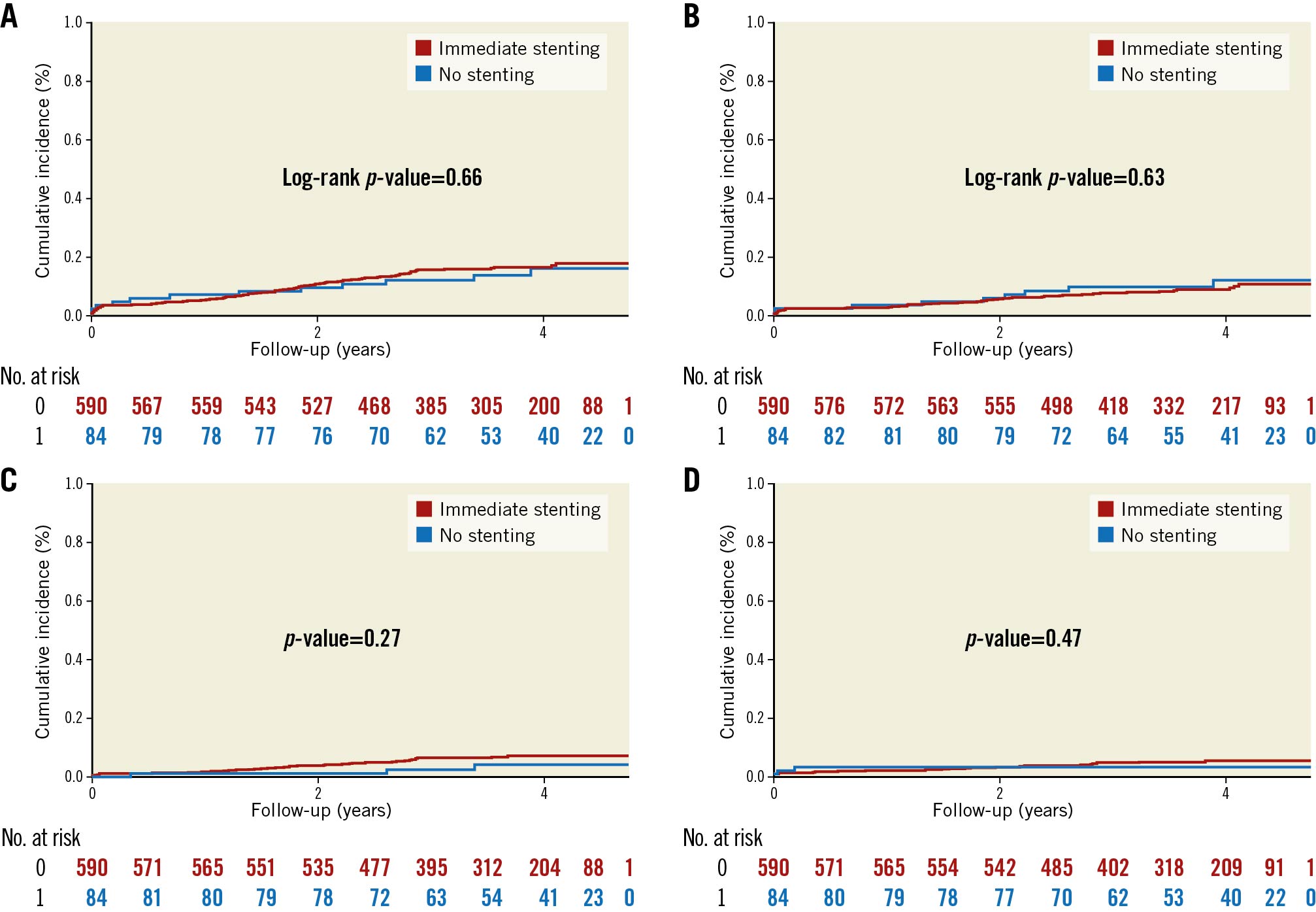
Figure 2. Cumulative incidence of (A) the primary composite endpoint, (B) all-cause mortality, (C) recurrent MI, and (D) TVR during follow-up, in STEMI patients treated with no and immediate stenting. The cumulative incidence of the endpoints (A) the primary composite endpoint and (B) all-cause mortality were compared using the log-rank method. The cumulative incidence of the endpoints (C) recurrent MI and (D) TVR were calculated taking the competing risk of death into account. Gray’s test was used to compare groups for significant differences. The primary endpoint was a combination of all-cause mortality, recurrent MI, and TVR. The y-axis presents the cumulative incidence, and the x-axis presents follow-up time in years. The red graph presents patients with STEMI treated with immediate stenting, and the blue graph presents patients with STEMI treated with no stenting. Numbers at risk for each treatment group are given below the graph. MI: myocardial infarction; No.: number; STEMI: ST-segment elevation myocardial infarction; TVR: any unplanned target vessel revascularisation
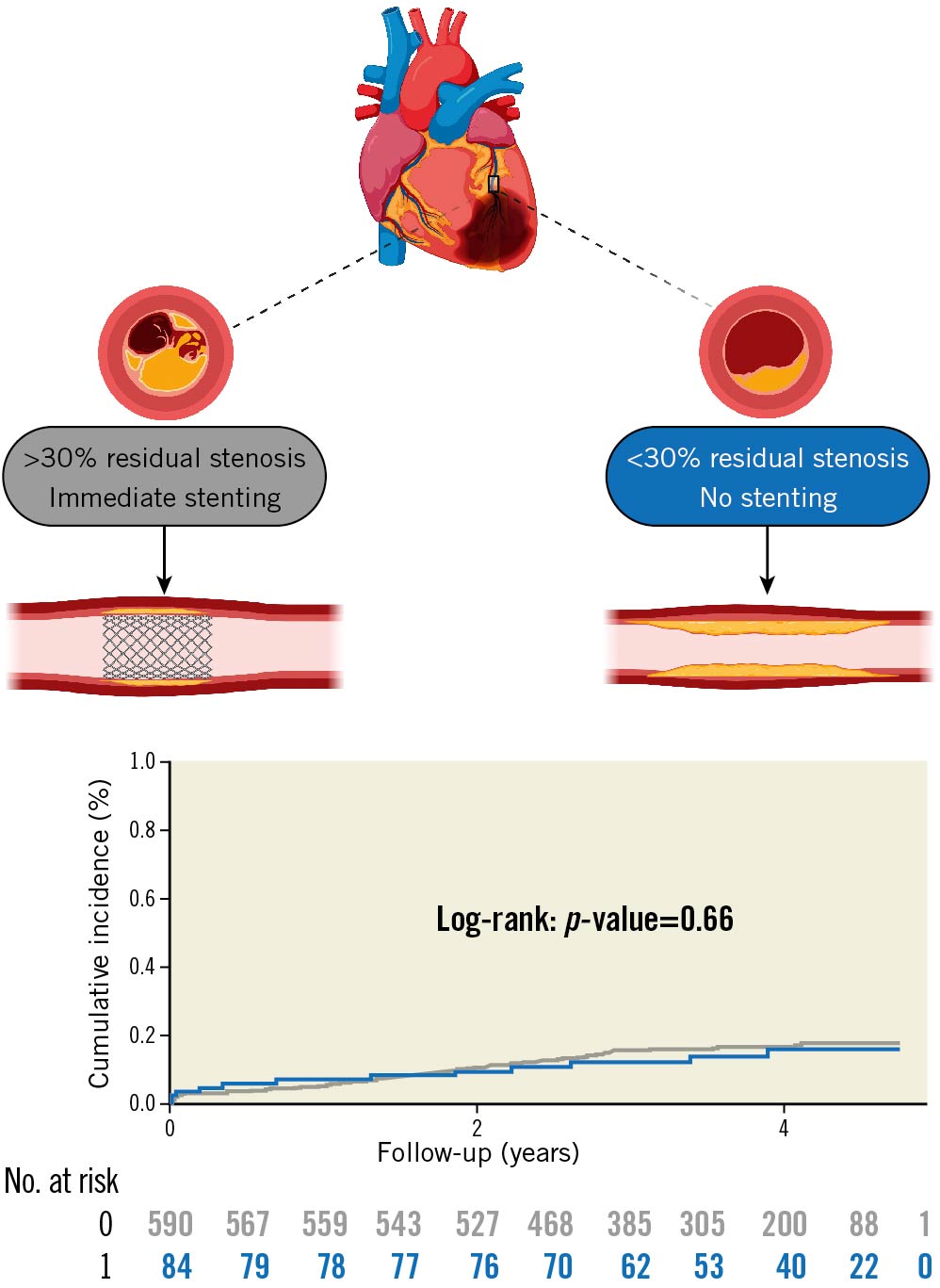
Central illustration. Cumulative incidence of the primary endpoint in patients with STEMI treated with no or immediate stenting. The top panel illustrates the rationale for omitting stenting in the no stenting group and stenting in the immediate stenting group. Below is a cumulative incidence curve of the primary endpoint. The primary endpoint was a combination of all-cause mortality, recurrent MI, and TVR. The y-axis presents the cumulative incidence, the x-axis presents follow-up time in years. The blue graph represents STEMI patients treated with no stenting. The grey graph represents STEMI patients treated with immediate stenting. Treatment groups were compared using the log-rank method. Numbers at risk are given below the graph. Illustration created with BioRender.com. MI: myocardial infarction; No.: number; STEMI: ST-segment elevation myocardial infarction; TVR: target vessel revascularisation
Supplementary analysis comparing the 4 groups of patients: 1) those treated with immediate stenting, 2) those treated with no stenting, 3) those treated with deferred stenting, and 4) those randomised to conventional treatment treated without stenting, showed no difference in event rates or cumulative incidence on the primary endpoint (Supplementary Table 2, Supplementary Figure 1). Furthermore, analysis showed no difference between the event rates of the primary endpoint in the no stenting group and patients treated with no stenting in the conventional group (Supplementary Table 3).
Discussion
In this post hoc analysis of the DANAMI-3-DEFER trial, patients treated without stenting, in general, had very low event rates in terms of all-cause mortality, recurrent MI, and TVR. The decision to omit stenting in the no stenting group was not random, but at the discretion of the operator, based on the parameters described in the method section. These patients were therefore selected, which is underlined by the difference in important baseline factors such as culprit lesion (which was more frequent in the left anterior descending artery in the immediate stenting group), diabetes mellitus, and multivessel disease. Also, patients in whom it was necessary to stent immediately in order to restore a stable coronary blood flow are excluded from this cohort, and thus, the no stenting approach is selected for lower-risk clinical settings. Nevertheless, our findings indicate that it is safe to omit stenting in STEMI patients without a flow-limiting underlying stenosis in the culprit lesion and if it is possible to restore a stable coronary blood flow without stenting. Based on the results in the present paper, it may be suggested that for some patients with no significant residual stenosis, no thrombus, one-vessel disease, and no diabetes mellitus, stent implantation may not be beneficial as a treatment option, considering the life-long risk of restenosis and stent thrombosis.
In accordance with guidelines2, primary PCI including stenting is recommended in patients with STEMI to secure stable coronary blood flow and prevent re-occlusion and restenosis. Deferred stenting is not implemented in clinical practice, as prior studies have suggested that deferring the stenting does not provide any clinical benefits compared to immediate stenting111520. However, there may be a potential clinical benefit of deferred stenting in selected patients, as it may prevent slow flow and distal embolisation21 and reduced infarct size 20. In the present post hoc study of DANAMI-3-DEFER, we have shown that the strategy of deferred stent implantation may lead to avoidance of stenting in patients with adequate flow and minimal residual stenosis. Our findings further suggest that it may be safe to omit stenting in these patients as event rates of TVR and recurrent MI were low. Patients treated with no stenting had a large thrombotic burden at the end of the procedure, but lesions in this group underwent minimal mechanical manipulation due to the no stenting approach, which may indicate an absence of peripheral embolisation, and therefore may offer the no stenting group an advantage. On the other hand, both treatment groups had a high level of post-PCI TIMI 3 flow, suggesting a low incidence of distal embolisation.
Stenting per se carries various risks of both acute and chronic intravascular complications, including stent thrombosis and in-stent restenosis56, but our study is too small to draw firm conclusions as to these events. Of note, endothelial function is severely damaged in the acute setting, causing inappropriate distal vasoconstriction. This may lead to a higher risk of stent thrombosis due to misjudgements of true vessel size and, consequently, stent underexpansion2223. Contemporary rates of stent thrombosis with modern drug-eluting stents are quite low. However, if present, mortality has been reported as high as 45% and reoccurrence up to 20%24. Also, stent implantation may initiate more chronic complications of progressive growth of the neointima in the coronary artery, causing in-stent restenosis24. Each patient has an individual risk of complications which may not be known until after the primary PCI. Nevertheless, the incidence of TVR and recurrent MI were, in general, low, and our multivariate analysis showed no association between no stenting and an increased risk of the composite endpoint, even after a median follow-up period of 3.4 years.
Rupture of a vulnerable coronary plaque and exposure of underlying thrombogenic substrates in the lipid core, triggering thrombus formation, is known to be the underlying pathophysiology of STEMI19. However, it is recognised that some of the ruptured plaques undergo spontaneous healing, modelling more stable atherosclerotic lesions, and thereby remaining clinically silent and stable25. Our study showed that patients with STEMI and less than 30% residual stenosis, who were treated with no stenting at the discretion of the physician, did not have a higher incidence of recurrent MI or TVR compared to patients treated with immediate stenting. However, 21% of the patients in the no stenting group did not undergo intervention, which may have influenced the prognosis of these patients positively. Nevertheless, these findings support the current knowledge that only ischaemia-causing lesions are associated with worse prognostic clinical outcomes26. Thus, patients with STEMI and stable coronary blood flow subsequent to the re-opening of the coronary artery may undergo a positive healing process with no stenting, implying comparable prognostic clinical outcomes to patients treated with immediate stenting. Additionally, the positive healing process may be further improved by dual antiplatelet therapy (DAPT) following STEMI, as DAPT possibly protects patients from recurrent thrombotic events27. Hence, in patients with STEMI and stable coronary blood flow, with minimal underlying stenosis and treated with no stenting, DAPT may be the primary contributor to positive plaque healing.
Since the introduction of drug-coated balloons, the randomised clinical trial, BASKET-SMALL 2 has investigated the safety and efficacy of drug-coated balloons in comparison to drug-eluting stents in small coronary arteries (<3 mm diameter). The trial found drug-coated balloons to be non-inferior to drug-eluting stents, persisting into the long term28. Furthermore, a recently published analysis of the trial showed lower events of TVR in diabetic patients treated with drug-coated balloons compared with drug-eluting stents29. Corresponding to these findings, our study suggests that refraining from stent implantation in the setting of STEMI in targeted patients with re-established adequate flow, but without significant underlying significant stenosis, may therefore not affect prognosis. This study is, however, of a post hoc nature, and our findings remain hypothesis-generating. Thus, the majority of patients treated with no stenting in our study were treated with POBA and not drug-coated balloons, but the findings are intriguing and merit future prospective research in alternative and better treatment strategies for no stenting in STEMI patients with no significant stenosis. Moreover, the discretion of the decision for no stenting in selected patients was driven purely by angiography. Thus, supplementary intravascular imaging could have potentially reinforced our results, since intravascular imaging could have evaluated the lesion stenosis, plaque characteristics, and lesion length more precisely. Lastly, but importantly, patients with STEMI are on average younger than patients with other subsets of ischaemic heart disease. Therefore in these younger patients, refraining from permanent implants is of distinct and major interest30.
Limitations
Due to the post hoc nature of this study, the findings may only be hypothesis-generating. Differences in antiplatelet and anticoagulant regimes between the two groups may have been a confounding variable in the findings of the study. Furthermore, the decision to omit stenting was not random. Patients treated with no stenting were randomised to deferred stenting during the primary PCI procedure, and had no significant residual stenosis and a stable coronary blood flow. The integrity of these findings in the study therefore only applies to patients with STEMI that fit these criteria, and omitting stenting does not account for all patients with STEMI. Also, the non-significant findings between patients randomised to conventional treatment but treated without stenting may have been due to lack of power. Despite differences between groups, the findings of the present study may, however, indicate that stenting may be safely omitted in patients meeting specific criteria.
Conclusion
Patients with STEMI, with no significant residual stenosis and stable coronary blood flow after initial PCI who were treated without stenting, had, in general, low rates of both the composite endpoint of mortality, recurrent MI, and TVR, and the individual components themselves. These were comparable to patients with STEMI treated with immediate stenting.
Impact on daily practice
STEMI is most often treated with stent implantation to secure coronary flow and prevent re-occlusion and restenosis even though the underlying stenosis is often not severe. Our findings suggest that it is safe to omit stenting in patients with STEMI if there is no flow-limiting underlying stenosis in the culprit lesion and a stable coronary blood flow can be restored. These findings are intriguing and appeal for future randomised clinical trials in alternative treatment strategies for no stenting in patients with STEMI and no significant stenosis.
Funding
This work was supported by the Alfred Benzon Foundation and the Novo Nordisk Foundation. The funders were not involved in any aspects of the study or the decision to submit for publication.
Conflict of interest statement
C. Terkelsen has received a research grant from Terumo, consulting fees from Edwards, Meril, Boston Scientific, and Bristol-Meyers Squibb, and lecture fees from Terumo, unrelated to this topic. L. Køber has received speaker’s honorarium from Novo Nordisk, AstraZeneca, Boehringer, and Novartis, and participated in Data Safety Monitoring of the INFINITY trial, unrelated to this topic. T. Engstrøm has received speakers fees from Abbott Vascular, Boston Scientific, and Bayer, an advisory board fee from Abbott, and participated in Data Safety Monitoring of the INFINITY Trial, unrelated to this topic. J.T. Lønborg has received an advisory board fee and an unrestricted grant from Boston Scientific, unrelated to this topic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

