Abstract
Aims: Disturbance in the flow of an infarct-related artery due to embolisation of thrombus and plaque material occurs frequently during primary percutaneous coronary intervention (PCI) and is associated with impaired prognosis. The aim of the present study was to minimise the risk of embolisation during PCI in patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results: Of 124 consecutive patients with STEMI, thrombectomy and/or balloon dilatation was performed in 110 (89%). Stent implantation was deferred in 113 (91%) patients who then comprised the study group. In 38% of the patients stent implantation was deemed unnecessary at the second examination because of <30% residual stenosis and no visible thrombus, and all lesions re-examined three months later were patent. Major adverse cardiac events occurred in two patients during eight months of follow-up (one cardiac death, one case of reinfarction with target lesion revascularisation). In five patients no PCI was performed at all. Myocardial salvage determined by cardiac magnetic resonance in a subset of patients was relatively high.
Conclusions: Deferred stent implantation is safe in the majority of patients with STEMI. Although the concept has to be evaluated in a randomised trial, the strategy may prove beneficial for many patients referred for primary PCI.
Introduction
Percutaneous coronary intervention (PCI), including thrombectomy and stent implantation, is the most efficacious treatment of patients with ST-segment elevation myocardial infarction (STEMI) as it reduces the occurrence of reinfarction and improves the prognosis in comparison with fibrinolytic treatment1-3. Stent implantation per se does not seem to alter the prognosis, and the indication for implantation of drug-eluting or bare metal stents in this type of patient is not clear-cut4-7. Despite apparently successful revascularisation of the epicardial part of the occluded vessel, distal embolisation occurs in 5-10% of patients which, like microvascular dysfunction, is associated with an impaired prognosis8-11. However, it is unknown whether disturbances in the microcirculation are entirely caused by distal embolisation from the ruptured plaque. Attempts to avoid embolisation by using distal protection devices have not proved efficacious12,13, and thrombectomy seems beneficial in some settings, but increases infarct size in others14,15. Reduced flow to the vascular bed of the infarct-related artery (IRA) is observed in a considerable number of patients treated with primary PCI, especially after stent implantation, even in patients with a normal epicardial flow9,16,17. Postponement of stent implantation (deferred stenting) for hours or days may potentially allow the thrombus material of the IRA to resolve considerably and thereby limit the risk of embolisation and improve the prognosis of the patient. On the other hand, the risk of reocclusion is increased in patients who are left unstented18. Other considerations include: 1) which of the procedures results in the least frequent need for repeat revascularisation; 2) stent thrombosis and; 3) costs. Since implantation of second-generation drug-eluting stents seems to indicate a very low level of stent thrombosis, it is important to evaluate any alternative treatment strategy contrary to contemporary optimal device and medical therapy.
The present study evaluates whether it is safe to defer stent implantation in patients with normal epicardial flow on admittance or after thrombectomy with or without small size balloon dilation of the culprit lesion combined with an optimal medical regimen of antiplatelet therapy. To evaluate the efficacy of the concept we recorded the clinical outcome, and in a subgroup of patients we measured the myocardial salvage index determined by cardiac magnetic resonance (CMR).
Methods
PATIENT POPULATION
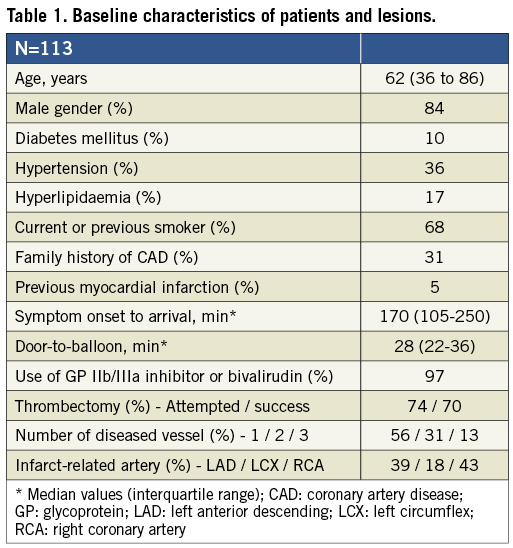
Patients referred for primary PCI due to chest pain and ≥0.2 mV ST-segment elevation in ≥2 contiguous electrocardiographic leads with a stable thrombolysis in myocardial infarction (TIMI) flow 3 (TIMI flow 2 in cases of very large vessels) obtained either spontaneously or after thrombectomy and/or balloon dilatation of an occluded coronary artery were considered as candidates for the study. The main exclusion criteria were type C dissections or worse of the culprit lesion, culprit lesions in unprotected left main coronary arteries or saphenous vein grafts, previous myocardial infarction in the target vessel area, stent thrombosis and gastrointestinal bleeding within one month. The clinical course was compared with that of a control group treated conventionally5. The study protocol was part of a larger randomised trial approved by the local ethics committee, and all patients gave their informed consent.
PRIMARY CORONARY INTERVENTION (FIRST PROCEDURE)
All patients were pretreated with 300 to 500 mg aspirin, 600 mg clopidogrel or 60 mg prasugrel, and 10,000 IU unfractionated heparin. Additional heparin (as indicated by measurements of the activated clotting time), bivalirudin bolus and infusion for four hours in reduced dose and/or glycoprotein IIb/IIIa receptor blockers, abciximab or eptifibatide, were administered during the PCI procedure (bolus and 12 or 16 hours infusion, respectively). Patients with unstable lesions or impaired blood flow of the IRA at admission had an acute PCI performed using wire introduction, thrombus aspiration using the 6 Fr Export® AP aspiration catheter (Medtronic, Minneapolis, MN, USA), and/or dilation in the lesion with an undersized balloon (1.5 or 2.0 mm in diameter). In patients with TIMI flow 3 at admission consideration was given as to whether PCI was necessary to obtain a stable blood flow. All equipment was used at the discretion of the operator, with the exception that stent implantation was only allowed in case of threatened occlusion or jeopardised blood flow in the IRA.
RE-ANGIOGRAPHY/INTERVENTION (SECOND AND THIRD PROCEDURE)
A re-angiography was planned 48 to 72 hours after the primary procedure, and stent implantation was performed in the culprit lesion in cases with a residual diameter stenosis >35% by visual estimate. Patients who did not have a stent implanted were offered a third angiography three months later. Clopidogrel 75 mg daily or prasugrel 10 mg for one year and aspirin indefinitely were prescribed for all patients. TIMI flow of the IRA and diameter stenosis of the infarct-related lesion were evaluated independently and the severity of thrombus burden classified as previously described19.
CARDIAC MAGNETIC RESONANCE IMAGING
CMR imaging was performed in the last 30% of patients consecutively included at Rigshospitalet, Copenhagen, in line with this examination being introduced as a routine procedure in patients with STEMI, as previously described on a 1.5 T scanner (MAGNETOM® Avanto or Espree scanner; Siemens, Erlangen, Germany)20. Briefly, an initial scan was performed within two days after primary PCI to assess the myocardial area at risk (oedema) with a signal intensity higher than two standard deviations above the signal intensity of the normal myocardium, using a T2-weighted short-tau inversion-recovery sequence21-23. A second scan was performed approximately three months later in order to assess the final infarct size using delayed-enhancement imaging. Final infarct size was identified using a 5-standard deviation threshold and was expressed as % of left ventricular mass24. The salvage index was calculated as (area at risk minus infarct size)/area at risk. LV ejection fraction was assessed using an ECG-triggered balanced steady-state free precession cine sequence. LV volumes were measured by manually tracing the endocardial borders throughout the cardiac cycle with automatic identification of end-diastolic and end-systolic time frames.
EVENTS AND DEFINITIONS
The clinical course was evaluated in all patients during a six- to 15-month follow-up period (median eight months). We recorded the occurrence of premature stent implantation (stent implantation before scheduled), major bleedings and the occurrence of major adverse events (MACE) defined as cardiac death, recurrent myocardial infarction and clinically driven target lesion revascularisation (TLR).
Any death not clearly attributable to a non-cardiac cause was classified as cardiac.
Statistical analysis
Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables were compared using the Wilcoxon test for paired and the Mann-Whitney U-test for unpaired data. The Kaplan-Meier method was used to create survival estimates and statistical comparison was made using the Log rank test. All p-values were two-sided, and a p-value <0.05 was considered statistically significant.
Results
PROCEDURE
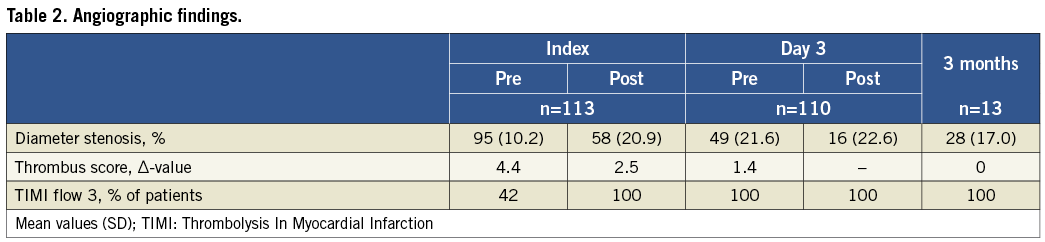
In 124 consecutive patients, immediate stent implantation was judged necessary in 11 (9%) because of threatened closure due to either recoil or dissection of the vessel wall in the infarct-related lesion (Figure 1). One of these was performed 15 minutes after the index procedure due to secondary closure of the vessel despite TIMI 3 flow at the end of the procedure and administration of bivalirudin. Thus, deferred stent implantation could be achieved in 113 patients (91%). The demographic characteristics of the study patients are outlined in Table 1. In 47 patients (42%) the IRA had TIMI flow 3 at the acute angiogram, and in 14 (12%) no immediate PCI was deemed necessary. Thus, acute PCI was performed in 99 patients (88%) with thrombectomy in 83. Angiographic and procedural data, including changes in thrombus burden, are presented in Table 2.
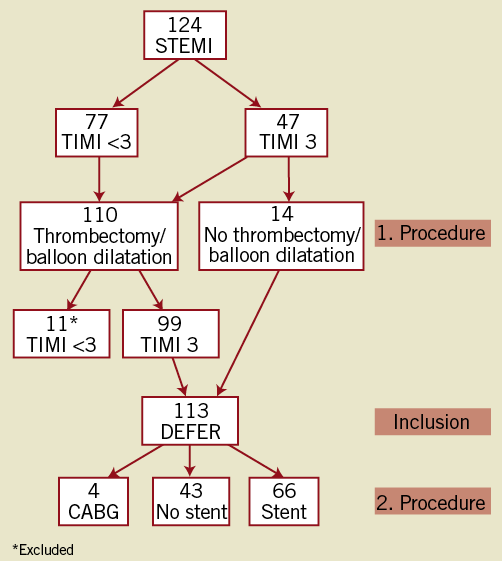
Figure 1. Flow chart of patients. During the first procedure patients were excluded in case TIMI 3 flow was not obtained. During the second procedure stents were implanted in case of residual stenoses >35%.
SECOND PROCEDURE
The IRA was patent in all patients who remained clinically stable after the index procedure. Of the 14 patients who had only an angiogram performed as the index procedure, stent implantation was deemed unnecessary in five at the secondary examination 24 to 72 (median 60) hours later and, in the remaining nine patients, stents were implanted in seven, while CABG was performed in two patients (due to multivessel disease). Of the 99 patients who had PCI performed at the index procedure, two were scheduled for surgery and 59 had a stent implanted at the second invasive procedure. Thus, 66 patients had a stent implanted due to >35% residual diameter stenosis in the infarct-related lesion, whereas 43 patients did not. No significant differences were found in baseline characteristics between patients who had and patients who did not have a stent implanted. All patients had no or minimal residual thrombus at the second examination (Figure 2). The mean residual stenosis was considerably reduced from the index to the second procedure (Table 2).
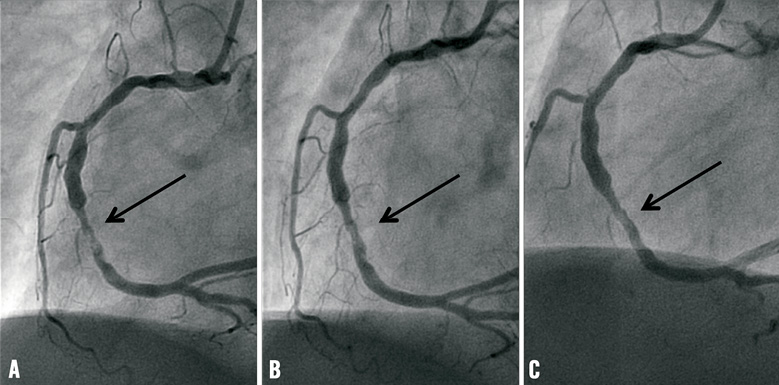
Figure 2. Patient with inferior wall STEMI in whom no PCI was performed. A) RCA at baseline showing irregular thrombus containing a lesion in the mid-RCA (arrow) with normal flow to the peripheral vascular bed, B) the same lesion after three days, and C) after three months.
THIRD PROCEDURE
A third angiography after three months was consented to by 13 of the 43 patients (30%) who had no stent implanted. The IRA remained patent in all these patients with no deterioration of the diameter stenosis or recurrence of thrombus, and stent implantation was deemed unnecessary in all of them (Table 2 and Figure 2).
CARDIAC MAGNETIC RESONANCE IMAGING
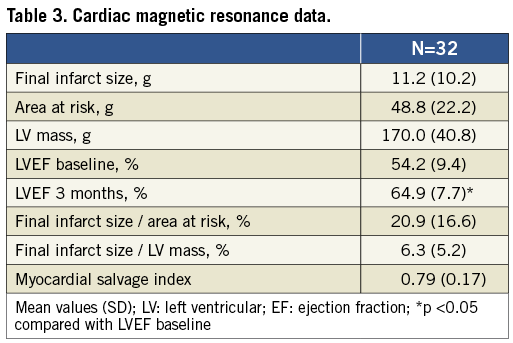
CMR was performed in the last 32 patients (28%) included in the study. The final infarct size ranged from 0% to 17% of the LV mass with a mean of 6.3% (Figure 3). The mean salvage index was 0.79 (range 0.38 to 1.00), and LV ejection fraction increased from 54% (range 35% to 77%) immediately after the index PCI, to 65% (range 49% to 80%) at three months (Table 3).
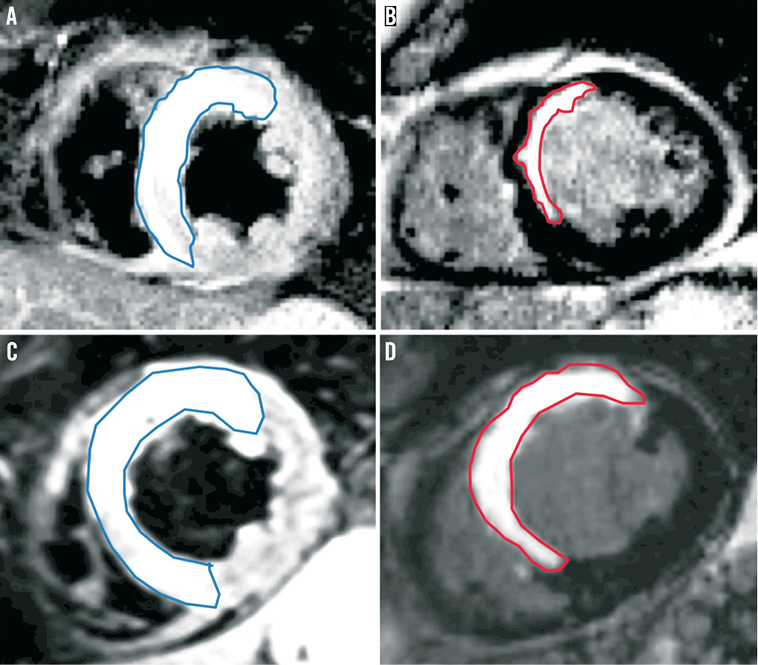
Figure 3. CMR images of two patients with STEMI in the territory of the left anterior descending artery. Left panels (A and C): T2-weighted short-tau inversion-recovery sequence imaged 24 hours after primary PCI to delineate area at risk (blue outline). Right panels (B and D): relatively small (B) and relatively large (D) final infarct size three months later using delayed-enhancement imaging (red outlining).
EVENTS
Clinical follow-up was complete in all patients. One patient had a haematoma at the access site after the index procedure requiring blood transfusion; however, no MACE occurred in any of the patients during the hospital stay. Four patients died, one after 13 days due to progressive heart failure, and three patients suffered a non-cardiac death (two patients due to lung cancer and one due to a ruptured abdominal aortic aneurysm). One patient with a bare metal stent implanted suffered a non-ST-segment elevation myocardial infarction after 120 days (in-stent restenosis) and TLR was performed. Thus, the MACE-free survival of patients treated with deferred stent implantation in this study was high compared with patients treated conventionally in a previous study6 (Figure 4).
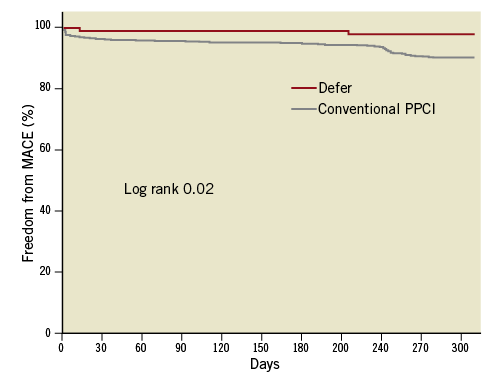
Figure 4. Kaplan-Meier estimates of MACE-free survival of patients treated with conventional primary PCI in a previous trial (grey line)6, and patients treated with deferred stent implantation in the present study (red line).
Discussion
We hereby present the angiographic and clinical outcome of an alternative deferred strategy to seal the infarct-related lesion rather than immediate stent implantation in patients with STEMI undergoing primary PCI. In nearly 40% of the patients it was deemed unnecessary to implant a stent in the infarct-related lesion at the second examination performed two to three days later. In the subset of patients without stent implantation, who had a control angiogram performed three months later, the IRA was found patent with no significant stenosis. In the initial phases of the alternative treatment leaving out stent implantation completely, we felt it necessary to make this control examination in a certain number of patients. On the other hand, a re-angiography should not be considered a standard part of the deferred stent concept.
Since a normal TIMI blood flow in the IRA after primary PCI is a prognostic indicator of the clinical course in patients with STEMI, the primary goal of the present study was to maintain a normal blood flow25. In some patients a normal blood flow of the IRA was present at arrival due to spontaneous recanalisation of the thrombotic occlusion, and in others it was obtained after thrombectomy and/or balloon dilation. To avoid microvascular flow disturbance, the lesion was left untreated without stent implantation in the acute phase. A stable blood flow of the IRA was obtained in all our patients after the index procedure (angiography and/or PCI), and no deterioration of blood flow was recorded in any patient after the second procedure. Although the present pilot study does not allow this surrogate marker of success to be directly translated into an improved clinical course, we did find a low rate of subsequent events in our patients. The MACE rate at eight months was lower in patients who had deferred stent implantation, compared with the rate in patients treated conventionally5, and both cardiac mortality and the combination of cardiac death and reinfarction were lower than reported in other trials2,8,15.
One of our 124 patients had subacute closure of the culprit artery immediately after the initial treatment, despite the judgement of the operator that the lesion and flow were stable, and bivalirudin was administered. It remains to be evaluated whether the risk of reocclusion in the period between the index and deferred procedure, in addition to potential complications related to an additional invasive procedure including bleeding at the access site(s), is outweighed by a lower risk of embolisation and flow disturbances of the peripheral vascular bed of the IRA with subsequent impairment of LV function and ensuing development of heart failure. We recommend both administration of either a glycoprotein IIb/IIIa antagonist or bivalirudin, and retraction of the wire followed by several (5 to 10) minutes of observation to assure stability of the lesion and flow before terminating the primary PCI.
A two-step approach with delayed finalising of primary PCI in order to minimise microvascular obstruction has been conducted in a few small trials26-28. The results of these trials indicate a higher success rate without any risk of intercurrent vessel occlusion in patients with initial TIMI 3 flow, in whom stent implantation is deferred for 24 hours. Our study results corroborate those findings and extend the concept to the majority of patients presenting with STEMI and occlusion of the IRA at arrival. On the other hand, considerable differences in the referral pattern for cardiac surgery in patients with multivessel disease may lead to significant differences in long-term patient outcomes26.
Deferred stent implantation in patients with STEMI represents an alternative concept that might seem more cumbersome and expensive, especially for patients for whom a conventional treatment –including immediate stent implantation– would have been successful. On the other hand, microvascular obstruction may occur even in the presence of a normal epicardial blood flow and, apart from a large thrombus burden in the IRA, we lack indicators or predictors for embolisation and induction of no flow/slow flow occurring in a considerable number of STEMI patients in connection with stent implantation29-31. Thrombectomy and distal protection may help to preserve microvascular flow in the presence of visible thrombus in the IRA, although the clinical improvement using these approaches has proved of limited value12-15. Thus, at present, it is impossible to select patients who will benefit from a deferred strategy compared with a conventional stent implantation strategy.
Although delayed PCI resulted in a reduced thrombus burden and a lower frequency of distal embolisation and flow disturbances compared with immediate PCI in a small study of patients with STEMI and normal blood flow in the IRA at arrival, no difference was found in the LV ejection fraction or in the rate of in-hospital clinical events in the two groups27. In our patients, the residual stenosis and thrombus burden was considerably reduced during the antithrombotic regimen between the index and secondary procedure, and only two patients (6%) examined with CMR had a LV ejection fraction <50% after three months. Although our results seem promising, it is too early to implement the method in general because it is unknown whether the beneficial effect of the deferred stent implantation strategy will result in improved long-term outcome for all patients with STEMI.
Administration of glycoprotein IIb/IIIa antagonists and bivalirudin during primary PCI both seem to improve patency of the IRA and to reduce mortality32,33. We left it to the operator’s discretion to choose between these alternatives as long as a recommended regimen of the drugs was followed, including intravenous infusion after the index PCI.
A certain level of selection bias towards a low-risk treatment group in our study cannot be ruled out, since patients without a normal blood flow in the IRA after the index procedure were not included. Thus, our results only pertain to patients in whom a stable TIMI 3 blood flow can be obtained without acute stent implantation. On the other hand, approximately 60% of the patients had a totally occluded IRA at arrival, which corresponds to the rate observed in other trials2,7,12,13. It should be stressed that the CMR data are derived from a subset of patients, and that they should be confirmed in a larger trial.
Conclusion
We conclude that the risk of reduced flow to the vascular bed of the IRA can be limited by applying a deferred stent implantation strategy during primary PCI. Although the concept needs to be evaluated in a randomised setting, it seems both safe and efficacious resulting in a low rate of MACE, and high level of myocardial salvage determined by CMR. In order to evaluate the efficacy of deferred stent implantation in comparison with conventional primary PCI and postconditioning, we have initiated a third large-scale randomised trial (the DANish trial) to improve treatment of patients with acute myocardial infarction (the DANAMI-3 trial - Clinical Trials.gov no 01435408).
Acknowledgements
This research is supported by the Danish Agency for Science, Technology and Innovation and by the Danish Council for Strategic Research (EDITORS: Eastern Denmark Initiative to Improve Revascularisation Strategies, grant 09-066994)
Conflict of interest statement
The authors have no conflicts of interest to declare.

