Abstract
Aims: Data are limited on the prognostic impact of stent thrombosis and in-stent restenosis in patients treated with coronary stents. We examined the prognostic impact of stent thrombosis and in-stent restenosis in patients treated with percutaneous coronary intervention (PCI).
Methods and results: All patients who underwent stent implantation from 2002 to 2005 were identified in the Western Denmark Heart Registry. The hazard ratio (HR) for death associated with stent thrombosis or in-stent restenosis was estimated with a Cox regression analysis with stent thrombosis or in-stent restenosis as time-dependent variables. A total of 12,277 patients were treated with stent implantation. Stent thrombosis was observed in 111 (0.9%) patients and in-stent restenosis in 503 (4.1%) patients within 12 months after the index PCI. Occurrence of stent thrombosis was associated with an increased risk of death (HR=2.71 [95% CI: 1.72-4.27]) compared to cases without stent thrombosis. In-stent restenosis had no substantial impact (HR=1.17 [95% CI: 0.79-1.75]). However, in-stent restenosis presenting as non-ST-segment elevation myocardial infarction (NSTEMI) was associated with a greater mortality risk compared with presentation of in-stent restenosis without myocardial infarction (HR=3.11 [95% CI: 1.08-8.69]; p=0.036).
Conclusions: The occurrence of stent thrombosis and in-stent restenosis presenting with NSTEMI increased the mortality risk threefold whereas in-stent restenosis without myocardial infarction was not associated with an increased mortality risk.
Introduction
The long-term effectiveness and safety of coronary stents in everyday clinical practice (i.e., outside clinical trials) need continuous assessment1-5. Stent implantation is followed by a measurable but relatively small number of stent failures, including acute and late stent thrombosis or in-stent restenosis. Stent thrombosis is a serious complication of percutaneous coronary intervention (PCI), usually presenting as a myocardial infarction (MI)6-8.
Data are limited on the prognostic impact of definite stent thrombosis and in-stent restenosis in patients treated with drug-eluting (DES) or bare metal stents (BMS)9-12. In the BMS era, both stent thrombosis and in-stent restenosis were associated with increased mortality12. In patients presenting with a stent thrombosis, the recurrence rate of a secondary stent thrombosis is high and confers a poor prognosis9.
The aims of the present study were to assess: 1) the risk of definite stent thrombosis or in-stent restenosis occurring within 12 months after an index PCI; 2) the clinical presentation of definite stent thrombosis or in-stent restenosis in stent-treated patients; 3) the prognostic impact of definite stent thrombosis or in-stent restenosis within 12 months after the index PCI in terms of all-cause mortality.
Patients and methods
SETTING AND DESIGN
We conducted the study using Western Denmark’s healthcare databases, which cover the region’s entire population (approximately 3.0 million inhabitants; 55% of the Danish population). All patients were followed for 36 months after the index PCI. A detailed description of the databases has been published previously13.
PATIENTS AND PROCEDURES
Using the Western Denmark Heart Registry (WDHR), all PCI procedures recorded between 1st January 2002 and 30th June 2005 were identified (n=12,922). Patients treated with balloon angioplasty or a combination of a BMS and a DES (n=645 [4.9%]) were excluded. Thus, the study population was patients treated with either a BMS or a DES (n=12,277), who presented with either a definite stent thrombosis or a clinically-driven in-stent restenosis within the first 12 months after the index procedure. All patients were followed for another 24 months after the event (Figure 1). After the index procedure, antiplatelet regimens included lifelong acetylsalicylic acid (75-150 mg daily) and clopidogrel with a loading dose of 300 mg followed by 75 mg daily. Since November 2002 the recommended duration of clopidogrel treatment has been 12 months for all stent types.
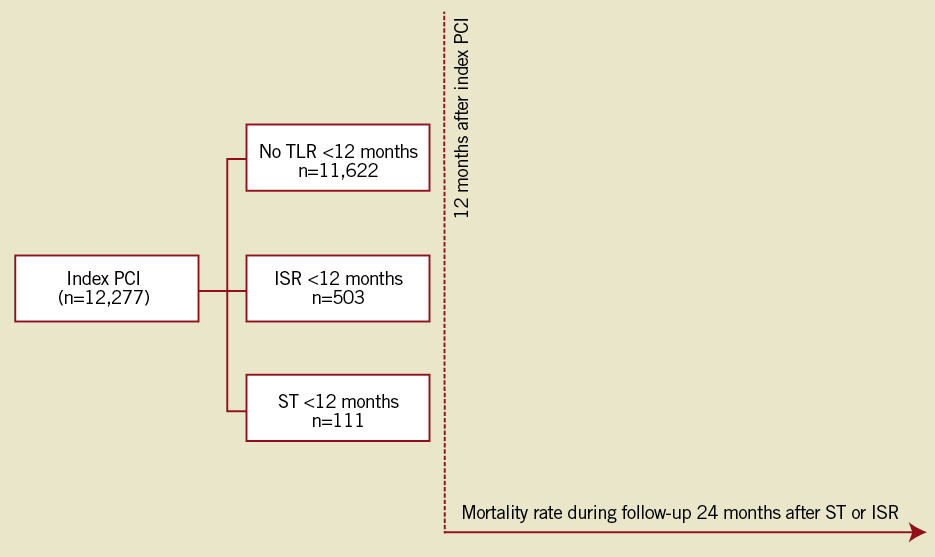
Figure 1. Flow chart for all patients treated by percutaneous coronary intervention with either a bare metal stent or a drug-eluting stent from January 2002 to June 2005, who then experienced definite stent thrombosis, in-stent restenosis or no target lesion revascularisation.
We retrieved relevant medical files and angiographic recordings for the target lesion revascularisation (TLR) patients.
VARIABLE DEFINITIONS
The main predictors of mortality we assessed were definite stent thrombosis and in-stent restenosis.
STENT THROMBOSIS
We characterised stent thrombosis using the Academic Research Consortium (ARC) definition14. Definite stent thrombosis had to be confirmed angiographically and accompanied by one or more of the following signs within 48 hours: new onset of ischaemic symptoms at rest, new electrocardiographic changes suggestive of acute ischaemia, or typical rise and fall in cardiac biomarkers. Stent thrombosis was further characterised according to time of occurrence after the PCI, i.e., as acute (0-<24 hours), subacute (≥1 day-<30 days), late (≥30 days-<1 year) and very late (≥1 year-24 months).
IN-STENT RESTENOSIS
In-stent restenosis was defined as a stenosis of more than 50% (visually assessed) within the stent or within a 5 mm border proximal or distal to the stent in patients with symptoms. Angiographic follow-up was not scheduled, instead patients were re-admitted due to symptoms and TLR was clinically driven. All in-stent restenosis were symptomatic in-stent restenoses.
MYOCARDIAL INFARCTION (MI)
We defined a new MI as a hospitalisation for MI. All admissions and re-admissions for MI were ascertained from the Danish National Patient Registry (ICD-10 codes I21-I21.9)15, which maintains records on all hospitalisations in Denmark. The myocardial infarction endpoint was defined according to the universal definition used by the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the World Heart Federation16. All relevant records (including electrocardiogram [ECG] and biomarkers) were reviewed by a clinical events committee consisting of at least two members from each participating hospital.
DEATH
We ascertained deaths from the Danish Civil Registration System17 and from the Danish Registry of Causes of Death.
OTHER POTENTIAL PREDICTORS
From the WDHR, data on potential predictors of subsequent cardiovascular events were retrieved. From the Danish National Patient Registry we obtained data for each patient on all hospital diagnoses and computed comorbidity scores using the Charlson comorbidity index18, which covers 19 major disease categories, including diabetes mellitus, heart failure, cerebrovascular diseases and cancer. The Charlson comorbidity index score is a weighted summary of the diagnoses, with weights based on the one-year mortality associated with each disease in the original Charlson dataset18.
CLINICAL EVENT DETECTION
Data on coronary angiography, repeat percutaneous coronary intervention, coronary bypass surgery, hospital admission and mortality were obtained for all patients from the following national Danish administrative and healthcare registries: the Danish Civil Registration System; the WDHR13; the Danish National Registry of Patients15, which maintains records on all hospitalisations in Denmark; and the Danish Registry of Causes of Death19.
From the WDHR we ascertained the occurrence of TLR, defined as a repeat PCI of the index lesion or coronary artery bypass grafting (CABG), occurring within one and three years following the index stent implantation. For all cases of in-stent restenosis and stent thrombosis we reviewed relevant medical records and catheterisation films, and a clinical events committee reviewed relevant records and adjudicated the endpoints regarding in-stent restenosis and stent thrombosis.
The Danish National Health Service provides universal tax-supported health care, guaranteeing residents free access to general practitioners and hospitals. The Danish Civil Registration System has kept electronic records on gender, birth date, residence, emigration date, and vital status changes since 196820, with daily updates; the 10-digit civil registration number assigned at birth and used in all registries allows accurate record linkage. The Civil Registration System provided vital status data for our study participants and minimised loss to follow-up.
STATISTICAL ANALYSIS
We used the life table method to compute the 12-month (from index PCI) cumulative incidence of stent thrombosis and in-stent restenosis. We constructed Kaplan-Meier curves for patients with stent thrombosis and in-stent restenosis. A Cox regression analysis was used to estimate hazard ratio (HR) for mortality with stent thrombosis and in-stent restenosis as time-dependent variables. Follow-up began at the index procedure PCI time and for the time-dependent variables (stent thrombosis and in-stent restenosis) the follow-up started when one of these events occurred in order to assess the prognostic impact on mortality in the stent population. In all regression analyses, we included age, sex, diabetes mellitus, clinical indication, procedure duration, number of stents and comorbidity, as well as stent length and the size of the reference vessel in the lesion-specific analyses. We used the likelihood test ratio to test if stent type (DES or BMS) had an influence on mortality. All analyses were carried out using SAS software version 9.13 (SAS Institute Inc., Cary, NC, USA).
STUDY POPULATION
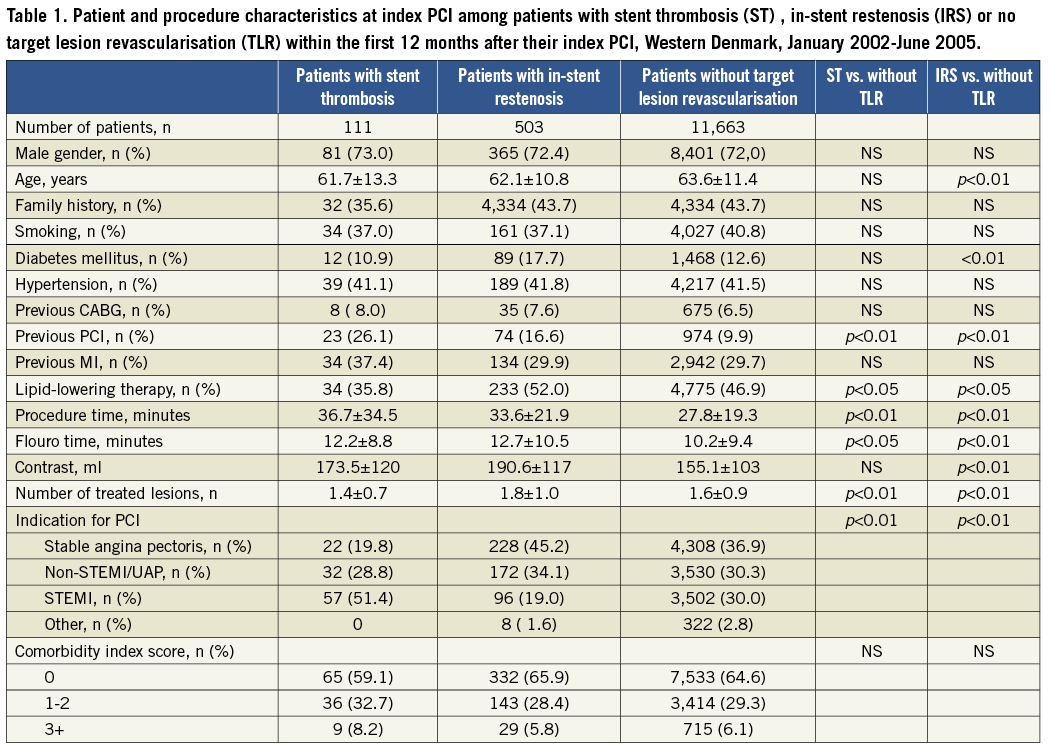
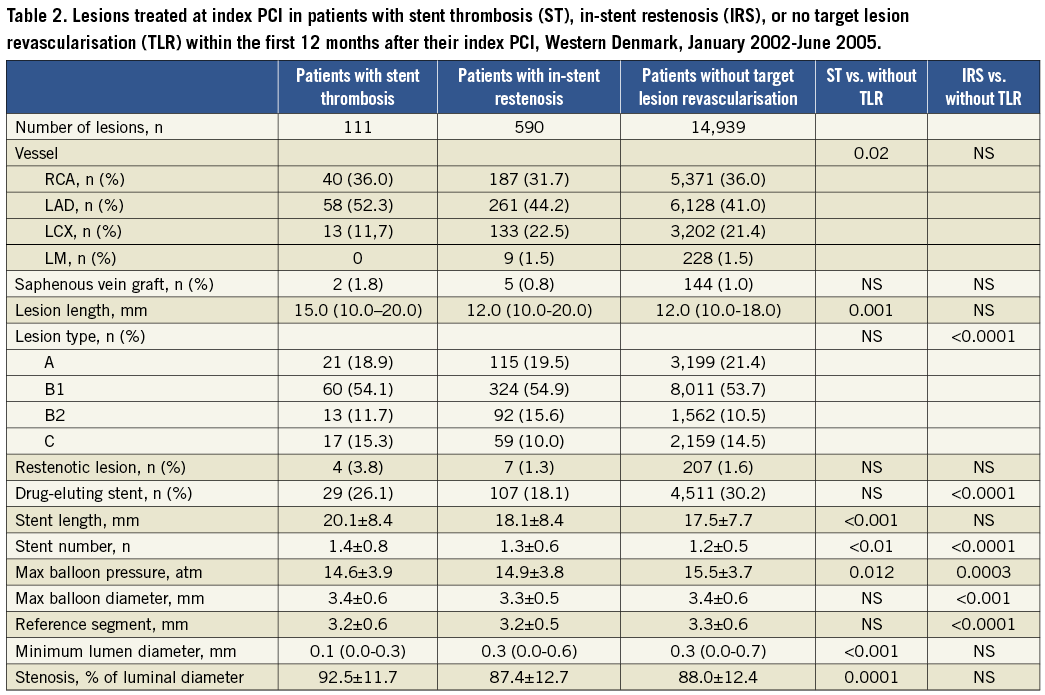
The study encompassed 12,277 consecutive patients. In these, a definite stent thrombosis was observed in 111 (0.9%) patients and an in-stent restenosis occurred in 503 (4.1%) patients within the first 12 months after the index PCI. At the index PCI, patients with a later definite stent thrombosis had a longer procedure time, flouro time, lesion length and stent length, but lower maximum balloon pressure compared to patients without TLR within the first 12 months after index PCI. Among patients with a definite stent thrombosis, the indication for the index PCI was more often ST-segment elevation myocardial infarction (STEMI), than among the comparison group. Patients with later in-stent restenosis also had longer procedure time, flouro time and higher contrast use, as well as smaller reference vessel size and lower maximum balloon pressure, compared to patients without TLR within the first 12 months after their index PCI (Table 1 and Table 2).
CLINICAL PRESENTATION AT THE TARGET LESION REVASCULARISATION PROCEDURE
Among patients with angiographically-confirmed stent thrombosis (n=111), 89.2% (n=99) were treated for STEMI and 10.8% (n=12) for causes other than STEMI. Patients with in-stent restenosis (n=503) were admitted due to stable angina pectoris (n=406 [80.7%]), unstable angina pectoris (n=59 [11.7%]), or non-STEMI (n=38 [7.6%]).
PROGNOSTIC IMPACT OF STENT THROMBOSIS AND IN-STENT RESTENOSIS ON MORTALITY AFTER PCI
For patients with a definite stent thrombosis within 12 months after the index PCI, the mortality rate after one, 12, and 24 months was 6.3%, 13.0%, and 17%, respectively (Figure 2). Compared to patients without TLR, a definite stent thrombosis within 12 months of the index PCI was associated with an increased risk of death (adjusted HR=2.71 [95% CI: 1.72-4.27]; p<0.001). The mortality rate did not differ significantly between patients with acute, early, or late definite stent thrombosis (Table 3).
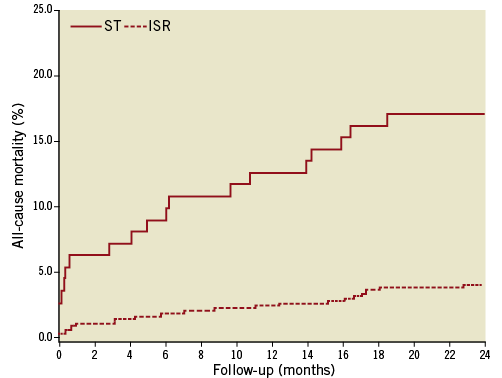
Figure 2. Relative risk of all-cause mortality in patients with a definite stent thrombosis or in-stent restenosis within 12 months after an index percutaneous coronary intervention with stent implantation.
Among patients treated for in-stent restenosis, the mortality rate after one, 12 and 24 months was 1.0%, 2.4% and 4.0%, respectively. There was no significant observed change in the risk of death among patients with in-stent restenosis compared to patients without TLR (adjusted HR=1.17 [95% CI: 0.79-1.75]; p=ns). In-stent restenosis with MI presentation was associated with significantly greater mortality risk compared with non-MI presentation (adjusted HR=3.11 [95% CI: 1.08-8.69]; p=0.036) (Figure 3). We found no difference in DES- vs. BMS-treated patients concerning 24-month mortality in patients with stent thrombosis or in-stent restenosis (p=0.41).
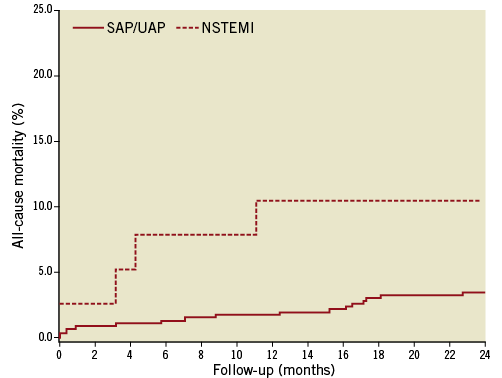
Figure 3. Relative risk of all-cause mortality in patients with a clinically-driven in-stent restenosis within 12 months after index percutaneous coronary intervention with stent implantation,
stratified after the clinical presentation with or without myocardial infarction at the time of in-stent restenosis.
Discussion
In a real-world clinical setting the rates of definite stent thrombosis or in-stent restenosis within 12 months were low. Most cases of definite stent thrombosis presented with STEMI, while nearly 20% of patients with in-stent restenosis presented with acute coronary syndrome (7% with non-STEMI and 12% with unstable angina pectoris), with no difference between BMS- and DES-treated patients. With three-year follow-up after index PCI of patients with a definite stent thrombosis or an in-stent restenosis 12 months subsequent to an index PCI, patients with a definite stent thrombosis had a mortality rate almost three times higher than patients without TLR. In contrast, in-stent restenosis did not overall predict mortality, whereas in-stent restenosis presenting with a non-STEMI increased the mortality risk.
The incidence of definite ST in our study was similar to the level reported in a meta-analysis6 of 10 randomised studies with nine to 12 months of follow-up comparing DES and BMS. It was also comparable to the results of the e-Cypher registry21. In comparison with randomised trials, the clinically-driven target lesion revascularisation of in-stent restenosis lesions in our study was markedly reduced22,23, but comparable to other registries without scheduled angiographic follow-up4,21. Clinical follow-up without angiographic monitoring reflects the normal pattern of patient care and is known to be characterised by less revascularisation than angiographically-monitored studies.
The present study demonstrates a more than twofold higher mortality rate in patients with definite stent thrombosis, compared to patients with STEMI treated with primary PCI, for which we have previously reported the two-year mortality rate24. The event of a stent thrombosis within 12 months after index PCI was more often seen in patients with an initial presentation of STEMI than in the other groups. This is in agreement with data from the Bern-Rotterdam registry25 and the WDHR1, where STEMI at the index time of stent implantation was found to be associated with later stent thrombosis. Our mortality rate is similar to the results recently reported by Ergelen et al10 in a study comparing outcomes after primary PCI for STEMI caused by stent thrombosis vs. de novo STEMI. They found that the long-term mortality rate was almost doubled in patients with stent thrombosis. Similarly, Ergelen et al observed that patients with stent thrombosis usually presented with STEMI, had had a previous PCI and had been treated for a left anterior descending artery (LAD) lesion at the index procedure. In agreement with our results, Van Werkum et al26 reported 30-day, one-year and two-year all-cause mortality rates (7.7%, 10.7%, and 12.0%, respectively) after definite stent thrombosis. We found that stent thrombosis had a prognostic impact as it was a strong predictor of death in patients with stent implantation. In contrast, clinical presentation of in-stent restenosis without MI was not associated with increased risk of death, whereas in-stent restenosis with MI presentation increased the mortality risk threefold. Doyle et al27 reported that stent thrombosis after BMS was associated with mortality when they performed a 10-year follow-up of patients treated between 1994 and 2000 in a single centre.
Furthermore, our study showed that patients with definite stent thrombosis had less lipid lowering therapy, longer procedure and flouro times, longer lesion and stent lengths, and lower balloon pressure. However, no significant difference in the maximum balloon diameter at the index PCI compared to patients without TLR could be demonstrated. These observations suggest that the index PCI was performed in lesions of high complexity.
In most of our study patients, the clinical presentation of stent thrombosis was STEMI. Overall, our study confirms that stent thrombosis is associated more often with a MI than in-stent restenosis. However, in patients with in-stent restenosis, we found that one out of five patients presented with acute coronary syndromes and one out of 14 had an MI. The in-stent restenosis rate was reduced with DES compared to BMS, through reduction of intimal hyperplasia developing over time. Intimal hyperplasia proliferation occurs over time and in-stent restenosis is thought to present as exertional angina. We observed no difference in the clinical presentation of in-stent restenosis in patients treated with DES vs. BMS.
The incidence of MI in patients presenting with an in-stent restenosis in our study is similar to the results of the study conducted by Chen et al28, who found that 9.5% of patients treated with BMS for in-stent restenosis presented with an MI. They found that 7.3% of patients presented with non-STEMI and 2.2% presented with STEMI. The latter may have been classified as a stent thrombosis in our study.
The validity of our findings depends on data quality and the ability to control for potential confounding. Our design is based on computerised registries with complete nationwide coverage, permitting study of a well-defined, large population with complete follow-up. We included only patients with an angiographically-confirmed definite stent thrombosis, according to the ARC definition and symptom-driven in-stent restenosis. Inclusion of probable and possible stent thrombosis would have increased the mortality rate further. Data on all key patient and procedure characteristics were >95% complete, and ascertainment of the main variables (stent thrombosis, death, MI and TLR) was 100% complete.
Conclusion
The occurrence of stent thrombosis and in-stent restenosis presenting with NSTEMI increased the mortality risk threefold in patients treated with coronary stent implantation whereas in-stent restenosis without myocardial infarction was not associated with an increased mortality risk.
Conflict of interest statement
The authors have no conflicts of interest to declare.

