Abstract
Aims: Stent thrombosis is a serious complication of percutaneous coronary intervention (PCI). We examined the incidence of stent thrombosis and other outcomes in patients treated with PCI and paclitaxel-eluting stents (PES), sirolimus-eluting stents (SES) or bare-metal stents (BMS).
Methods and results: All patients who underwent PES, SES or BMS implantation from January 2002 to June 2005 were identified in the population-based Western Denmark Heart Registry. All were followed for 36 months. Cox regression analysis was used to estimate relative risk (RR), controlling for covariates. A total of 12,374 patients were treated with stents: 1,298 with PES, 2,202 with SES and 8,847 with BMS. The three-year incidence of definite stent thrombosis was similar in the DES group (1.1%) and in the BMS group (0.7%) (adjusted relative risk [RR]: 1.24; 95% confidence interval [CI]: 0.85-1.81). Very late definite stent thrombosis occurred more frequently in DES-treated patients (adjusted RR: 2.89, 95% CI: 1.48-5.65). The three-year mortality rate did not differ significantly between the two groups. Target lesion revascularisation (TLR) was lower in DES-treated patients than in BMS-treated patients (adjusted RR: 0.71, 95% CI: 0.63-0.81).
Conclusions: An increased risk of very late definite stent thrombosis was observed in DES-treated patients compared with BMS-treated patients, but a similar mortality was detected. TLR continued to be lower among patients receiving DES.
Introduction
The long-term effectiveness and safety of drug-eluting stents (DES) in clinical practice (i.e., outside clinical trials) is controversial.1-5 Initially, the beneficial effects of DES did not seem associated with adverse effects on safety.6-8 Although DES continue to be widely accepted as safe and effective based on older randomised clinical trial settings,9,10 observational non-randomised studies have reported increased risks of stent thrombosis, myocardial infarction (MI), and death associated with DES compared to bare metal stents (BMS).3,11 The rate of late stent thrombosis associated with unrestricted DES use in real-world settings is 0.6% of patients per year.12
The class effect of DES treatment remains an important question in the BMS vs. DES controversy2, as there are major differences between the first commercial DES product, the sirolimus-eluting Cypher stent (SES), and the newer paclitaxel-eluting Taxus stent (PES).
In an earlier publication2, we observed an increased risk of very late definite stent thrombosis 24 months after DES implantation, with an annual rate of 0.2%, while the benefit of DES in reducing target lesion revascularisation was maintained. We now have extended the follow-up period to three years and have addressed the possible DES class effect on safety and effectiveness and also examined other predictors of stent thrombosis by reporting data separately for patients treated with SES, PES and BMS in Western Denmark.
Patients and methods
Setting and design
We conducted this follow-up study using Western Denmark’s healthcare registries and databases, which cover the region’s entire population (approximately 3.0 million inhabitants; 55% of the Danish population). All patients were followed for 36 months. A detailed description of the databases has been published previously13.
Patients and procedures
We used the Western Denmark Heart Registry (WDHR) to identify all percutaneous coronary interventions (PCI) recorded between January 1, 2002 and June 30, 2005. For each patient we included only the first PCI procedure performed during the study period (the index procedure). We excluded patients treated with balloon angioplasty or a combination of BMS and DES (n=645 [4.9%]). Post-PCI antiplatelet regimens included lifelong acetylsalicylic acid (75-150 mg daily) and clopidogrel with a loading dose of 300 mg followed by 75 mg daily. Since November 2002, the recommended duration of clopidogrel treatment has been 12 months for patients receiving all stent types (regardless of DES type).
Endpoints
Study endpoints were time to stent thrombosis, MI, all-cause mortality, cardiac death and target lesion revascularisation (TLR) as previously reported13. These events were ascertained from three sources: the WDHR; the Danish National Patient Registry (DNPR)14, which maintains records on all hospitalisations in Denmark; and the Danish Registry of Causes of Death15.
We characterised types of stent thrombosis using the Academic Research Consortium definition, with a modification for probable stent thrombosis.16
We defined a new MI as a hospitalisation for MI occurring >28 days after the index PCI.17 We ascertained admissions and readmissions for MI from the DNPR (ICD-10 codes I21-I21.9)14 and deaths from the Danish Civil Registration System.18 We validated the recorded cause of death using original death certificates obtained from the National Registry of Causes of Death, and classified deaths according to their underlying cause.15
From the WDHR we ascertained TLR, defined as a repeat PCI of the index lesion or coronary artery bypass grafting (CABG).
Covariates
From the WDHR, we retrieved data on potential confounders and other predictors of subsequent cardiovascular events. We also obtained data from the DNPR14 on all hospital diagnoses for each patient and computed comorbidity scores using the Charlson Comorbidity Index (CCI)19, which covers 19 major disease categories. Data on all key patient and procedure characteristics were >95% complete, and ascertainment of endpoints (stent thrombosis, death, MI and TLR) was 100% complete.
Statistical analysis
Distributions of continuous variables in the two groups (DES or BMS) were compared using either the two-sample t-test or the Mann-Whitney test. We compared distributions of categorical variables using the chi-square test.
Follow-up began on the date of the index PCI procedure. In analyses with stent thrombosis, MI or death as the outcome, follow up continued until the date of the respective medical event, death, emigration or until 36 months after implantation, whichever came first. We constructed Kaplan-Meier curves for patients and lesions treated with DES or BMS. We used the life-table method to compute the 36-month cumulative incidence for each endpoint (proportion of the population at risk with the outcome of interest). We used Cox proportional-hazards regression analysis to estimate relative risk (RR) for each endpoint. Since the hazards were not proportional throughout the follow-up period, we estimated RRs within time periods, during which the proportionality assumption held. The RR in these analyses reflected the risk among patients alive and at risk of a specific endpoint at the start of each time period (e.g., after 30 days or one year of follow-up). In all regression analyses, we included age, sex, diabetes mellitus, clinical indication, procedure duration, number of stents and comorbidity (and stent length and the size of the reference vessel in the lesion-specific analyses). All analyses were carried out using SAS software version 9.13 (SAS Institute Inc., Cary, NC, USA).
Results
Descriptive data
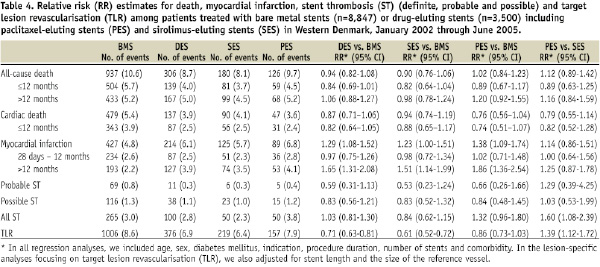
The study encompassed 12,347 consecutive patients with 17,146 lesions. Of these, 3,500 patients with 5,417 lesions received DES, either SES (n=3,426 [63.2%]) or PES (n=1,991[36.8%]), and 8,847 patients with 11,729 lesions were treated with BMS. Baseline patient, procedure, and lesion characteristics differed substantially between the DES and BMS groups, but were similar in the SES and PES groups (Tables 1 and 2).
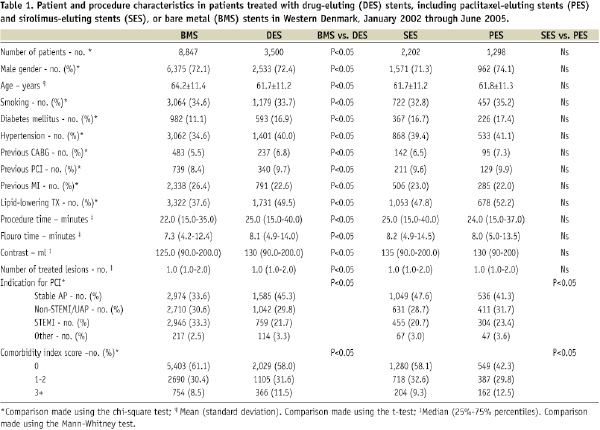
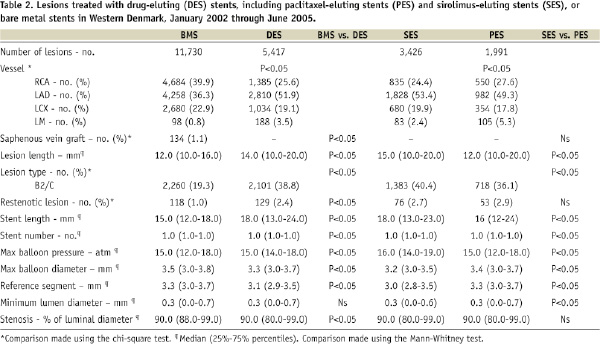
Stent thrombosis
DEFINITE STENT THROMBOSIS
During a follow-up period of three years, definite stent thrombosis occurred in 145 (1.2%) of 12,347 patients. The incidence of definite stent thrombosis did not differ substantially between the DES- and BMS-treated groups, with definite stent thrombosis occurring in 58 lesions in 58 patients treated with DES (3-year incidence=1.07%) and in 87 lesions in 87 patients treated with BMS (3-year incidence=0.74%) (adjusted RR: 1.24, 95% CI: 0.85-1.81) (Figure 1).
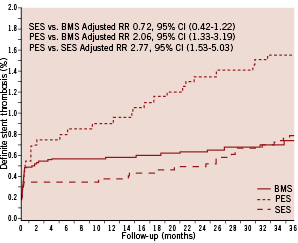
Figure 1. Crude risk of definite stent thrombosis among patients treated with SES vs. PES vs. BMS.
The incidence of acute, subacute and late definite stent thrombosis also was similar in the DES and BMS groups. Very late definite stent thrombosis occurred in 27 lesions in 27 patients in the DES group (3-year incidence=0.52%) and in 17 lesions in 17 patients in the BMS group (3-year incidence=0.15%) (adjusted RR: 2.89, 95% CI: 1.48-5.65) (Table 3).
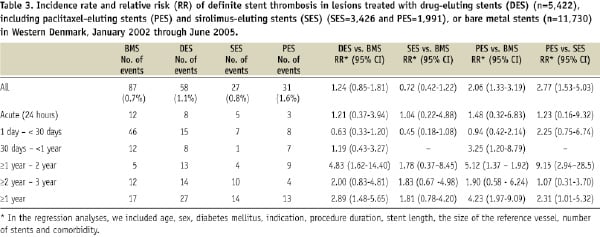
Early stent thrombosis occurred with similar frequency in the SES and PES groups (Figure 1, Table 3), but risks of very late stent thrombosis differed. Very late definite stent thrombosis occurred in 14 lesions in 14 patients in the PES group (3-year incidence= 0.70%) and in 14 lesions in 14 patients in the SES group (3-year incidence= 0.41%) (adjusted RR: 2.50; 95% CI: 1.10-5.67). The SES group did not differ significantly from the BMS group with respect to very late definite stent thrombosis, whereas the PES group had a substantially increased risk of this outcome (Table 3).
Predictors of overall definite stent thrombosis were ST-segment elevation myocardial infarction (STEMI) at the time of stent implantation (RR: 2.86, 95% CI: 1.83-4.48), stent length (RR per mm increase: 1.03, 95% CI: 1.00-1.05), use of PES (RR: 1.81, 95% CI: 1.16-2.84), age (RR: 0.97 per one year increase, 95% CI, 0.96-0.99) and reference vessel diameter (RR: 0.66 per mm increase, 95% CI: 0.47-0.93). Presence of diabetes mellitus did not predict definite stent thrombosis (RR: 0.87, 95% CI: 0.39-1.90). The only predictors of stent thrombosis occurring within 30 days were STEMI (RR 3.54, 95% CI: 1.92-6.50) and stent length (RR 1.03 per mm increase, 95% CI: 1.00-1.06). Factors associated with an increased risk of stent thrombosis after 30 days were STEMI (RR: 2.10, 95% CI: 1.07-4.10), age (RR: 0.96 per one year increase, 95% CI: 0.94-0.98) and use of PES (RR: 3.87, 95% CI: 2.08-7.18).
DEFINITE STENT THROMBOSIS AND ANTIPLATELET THERAPY
Among the 145 patients who developed definite stent thrombosis, 93 (64.1%) were being treated with dual antiplatelet therapy (aspirin and clopidogrel) at the time of the thrombotic event. In the 44 patients with very late stent thrombosis, four patients (9%) were receiving dual antiplatelet therapy, 34 patients (77%) were being treated with aspirin only, and six patients (14%) had discontinued both aspirin and clopidogrel.
Definite, probable or possible stent thrombosis
Definite, probable or possible stent thrombosis was found in 100 patients treated with DES (3-year incidence=2.8%), and in 265 patients treated with BMS (3-year incidence=3.0%). After controlling for covariates, the risk of stent thrombosis (definite, probable or possible) did not differ between the DES and BMS groups.
Mortality
All-cause (Figure 2) and cardiac 3-year mortality was lower among DES-treated than BMS-treated patients. After controlling for covariates, this difference disappeared (Figure 2, Table 4).
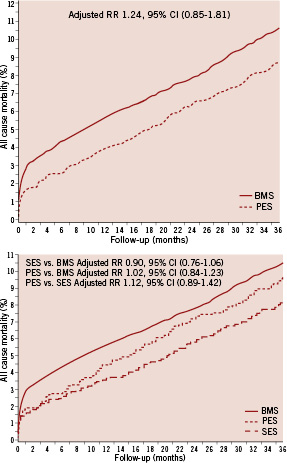
Figure 2. Crude risk of all-cause mortality among patients treated with PES, SES or BMS.
Mortality after definite stent thrombosis averaged 8.2% at one year: 11.1% among patients suffering from early definite stent thrombosis vs. 4.7% in patients suffering from late definite stent thrombosis (Figure 3). During the entire 3-year observation period, 21 patients suffering from definite stent thrombosis subsequently died. Death after the diagnosis of definite stent thrombosis thus occurred in 0.2% of the entire population and accounted for 1.7% of all 1,243 deaths.
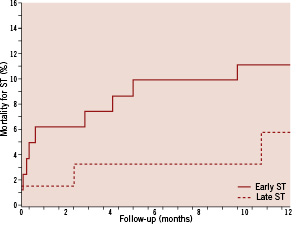
Figure 3. Crude risk of cumulative incidence of death after one year in patients (in days after definite stent thrombosis) with early (<30 days) or late (≥30 days) definite stent thrombosis.
Myocardial infarction
The 3-year incidence of MI was higher in the DES group (6.1%) than in the BMS group (4.8%). After controlling for covariates, the DES group continued to be at higher risk of MI (adjusted RR: 1.29, 95% CI: 1.08-1.52). This increase in MI risk was observed in both the SES and the PES groups compared to the BMS group (Table 4).
Target lesion revascularisation
TLR occurred less frequently among DES- than among BMS-treated patients (3-year incidence=6.9% vs. 8.6%). After controlling for covariates, the absolute risk reduction among patients in the DES group compared with the BMS group was 29% (adjusted RR: 0.71, 95% CI: 0.63-0.81). In the SES group the risk reduction was 39% compared to the BMS group (adjusted RR: 0.61, 95% CI: 0.52-0.72), and in the PES group the risk reduction was 14% compared to the BMS group (adjusted RR: 0.86, 95% CI: 0.73-1.03).
Discussion
In a real-world clinical setting with 3-year follow-up, patients treated with either DES or BMS had similar rates of definite stent thrombosis and mortality rates, while the DES group was at increased risk of MI. Moreover, the risk of very late definite stent thrombosis was elevated in DES-treated patients, mainly because of event rates in patients treated with PES. Finally, PES proved inferior to SES, and similar to BMS, in reducing TLR.
Very late definite stent thrombosis occurred at a steady annual rate of 0.2% in DES-treated patients, with no evidence of diminution after three years of follow-up. The rate of very late definite stent thrombosis found in our study was slightly lower than that in the Bern-Rotterdam Registry20; the latter reported a steady annual rate of 0.4% to 0.6% for up to four years. Among DES-treated patients included in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), an annual stent thrombosis rate of 0.5% was reported during two years of follow-up.5 One obvious difference between treatment strategies among patients followed in these three registries is duration of the antiplatelet regimen; patients in the WDHR received longer dual antiplatelet therapy compared to patients in the Bern-Rotterdam Registry20 and the SCAAR.5
STEMI at the time of stent implantation, stent length, use of PES, younger age and reference vessel diameter were other predictors of overall definite stent thrombosis, whereas STEMI, younger age and use of PES were associated with an increased risk of late stent thrombosis. The same three variables (STEMI, younger age and use of PES) were found to be associated with late stent thrombosis in the Bern-Rotterdam Registry.20
Registry data and meta-analyses21-24 compensate for the lack of adequately powered randomised clinical endpoint trials comparing BMS with DES, particularly by type of DES stent.25,26 When we compared SES and PES with BMS, use of PES was associated with more than a fourfold increase in very late definite stent thrombosis, whereas SES was associated with a non-significant 1.8-fold increase. The risks of definite stent thrombosis and very late definite stent thrombosis were elevated in PES-treated patients compared with SES-treated patients. Meta-analyses have reported a higher rate of very late definite stent thrombosis among patients receiving SES and PES, compared with those receiving BMS. Similar to the findings in our study, Kastrati et al22 found that PES, as compared to SES, significantly increased the risk of stent thrombosis and reintervention without significantly impacting risk of death.
Our data extend former research. In their meta-analysis of 38 randomised trials on BMS, PES and SES, Stettler et al found no significant differences in overall risk of definite stent thrombosis (0 days to four years), but the risk of late definite stent thrombosis (>30 days) was higher among patients receiving PES, compared to BMS (HR 2.11, 95% confidence interval 1.19-4.23) and SES (HR 1.85, CI 1.02-3.85).27 Bavry et al reported a similar finding21 in a meta-analysis of 14 contemporary clinical trials with patients randomised to PES or SES vs. BMS. Their observed rate of very late thrombosis was 5.0 events per 1,000 patients treated with DES and no events in patients treated with BMS (3.6 events per 1,000 SES-treated patients and 5.9 events per 1,000 PES-treated patients).
Until recently, DES was considered one stent type in registry studies13,28, and the effect of DES was considered a class effect. However, the drug release kinetics, the drug itself, and polymer or other characteristics of DES are likely to influence safety. The present study found that effectiveness differed between both DES types and BMS: TLR was reduced significantly in DES-treated patients compared to BMS-treated patients. However, within the DES subtypes, SES reduced TLR throughout the follow-up interval, while in PES-treated patients this association became weaker after three years. Similarly, Stettler et al’s meta-analyses23,29 reported a more pronounced reduction in TLR among patients receiving SES stents than among those receiving PES stents. The reintervention rate did not differ significantly between SES and PES stents within the first 24 months in randomised trials powered to evaluate this efficacy outcome.25,26 Although the SIRTAX trial30 showed that the Cypher-treated patients fared better than Taxus-treated patients at one year, this was no longer the case at five years. At the later time point there was significant difference in major adverse cardiac events (death, MI or TLR) between the two stents (stent thrombosis was not an endpoint in the SIRTAX study: Annual Scientific Symposium of Transcatheter Cardiovascular Therapeutics [TCT] 2009 – Late breaking clinical trial). Five-year results of the SIRTAX trial for mortality and MI are in line with our 3-year data. However, in contrast to the SIRTAX trial, we found a sustained significant reduction in TLR in SES treated patients after three years. A suggested “catch-up” phenomenon may exist with first-generation stents as a result of delayed vascular healing. Newer DES are being designed with the goal of enhancing safety, efficacy or both as compared with the previous devices. The evidence base for “next-generation” coronary stents has shown significantly reduced rates of stent thrombosis and TLR in the large-scale, prospective multicentre randomised SPIRIT IV trial (TCT 2009 – Late breaking clinical trial; comparing the everolimus-eluting Xience V stent to the paclitaxel-eluting Taxus Express stent) and in an all-comer population, COMPARE trial31, indicating that in real-world clinical settings, implantation of the everolimus-eluting Xience V stent significantly reduced major adverse cardiac events compared to the paclitaxel eluting Taxus Liberté stent. Superiority of the everolimus-eluting Xience V stent stemmed mainly from less early stent thrombosis and reduced TLR at one year follow-up.
Study limitations
Our observational study has several limitations. The validity of our findings depends on data quality and the ability to control for potential confounding. Our design is based on computerised registries with complete nationwide coverage, enabling study of a well-defined, large population with complete follow-up. Like all non-randomised studies, our study is prone to biases from non-random assignment of stent type and from uncontrolled confounding. However, we controlled for for a wide range of known confounding factors, our results may still be confounded by unmeasured factors like any subjective choice of the stent by the interventional cardiologist.
We collected data over a three-year period, during which the prevalence of DES stent use rose from zero to 53%. In order to reduce bias during this transition period, we followed every patient for 36 months.
Conclusions
Our study showed an increased risk of very late definite stent thrombosis and MI in patients receiving a DES compared to a BMS. Mortality, however, was similar in the two groups. Furthermore, among subgroups of patients treated with DES, a beneficial effect on TLR was maintained at 3-year follow-up for those receiving SES, but not for those receiving PES.

