Introduction
Results from the Swedish Angiography and Angioplasty registry (SCAAR) on outcome after implantation of drug eluting stents (DES) compared to bare metal stents (BMS) presented at the FDA advisory panel in December 2006, and subsequently published in the New England Journal of Medicine in February 2007, created great attention worldwide1. A possible 30% increase in late mortality with DES scared not only patients and physicians, but also health care providers and regulators. Interventional cardiologists had personally experienced sporadic cases of acute stent thrombosis and the thrombotic substrate, consisting of non-endothelialised stent struts, which had been convincingly demonstrated. An increased late mortality after DES in the large SCAAR study cohort was supported by findings from two recently presented meta-analyses of randomised trials, without access to patient level data2. The individual randomised trials did not show any increase in mortality but they were clearly underpowered for this evaluation.
With the addition of another year of patients from the SCAAR registry we demonstrated that DES did not seem to carry any increased long term risk. Delighted by this finding on behalf of millions of patients who had had DES already implanted, we presented the data at the European Society of Cardiology annual meeting in 2007. The results were referred to as «the Swedish Yo-Yo» in an ironical comment by Professor Serruys after the presentation, due to the initial signs of potential hazard with DES followed by more favourable results after inclusion of a larger cohort of subsequently treated patients.
Recently we published the outcome of patients enrolled over an extended four year period of time, with up to five years of follow-up in the New England Journal of Medicine and confirmed the safety of DES at long-term with an approximate 50% relative reduction of restenosis3.
What are the reasons for the relatively lower risk with DES in Sweden over time? Were the initial results incorrect and is there still any SCAAR scare?
SCAAR
The SCAAR registry includes all patients undergoing coronary angiography or percutaneous coronary intervention (PCI) in Sweden with a near 100% completeness of enrolment. Registration is performed by the treating physician on-line via a web-interface directly in the catheterisation laboratory. Over 100 variables are collected and the system creates procedure reports for clinical documentation. The system also offers a large series of reports about individual sites and doctors´ production and quality of care. For patients with previous interventions historic data are presented. An interactive method for registration of restenosis, mandating information about possible restenosis in every implanted stent at any subsequent coronary angiography or PCI, has been integrated in the registry since 2004 and, for stent thrombosis, since 2005. Thanks to the unique 10 digit personal identification numbers, and a possibility to link with other national registries, the follow-up is complete regarding death, myocardial infarction, bypass surgery and hospital care for any cause.
Monitoring and source-data verification of registry data has been performed in all hospitals since 2001 by comparing 50 entered variables in 20 randomly selected interventions per hospital and year with the patients’ hospital records. The overall correspondence is over 95%.
Change of patient characteristics over time
The most obvious change of patient characteristics over time was explained by a continuous increase in utilisation of primary PCI for ST elevation myocardial infarction during the study period. The proportion of patients with STEMI as an indication for stent implantation increased from less than 20% in 2003 to 30% in 2006. The increase was more attributed to the BMS group, as compared to the DES group. Over the years, also a larger proportion of patients have been pre-treated with higher doses of clopidogrel. Also, the acceptance of a prolonged duration of clopidogrel after stenting has increased in Sweden over the years, although, unfortunately, without the possibility of tracking this in the data base. These gradual changes in the treated populations and the simultaneous changes in preventive medications may have contributed to a relatively better outcome for patients receiving DES in later years, although none of theses changes alone can be proved to explain this relatively better outcome. With accumulating experience and knowledge about DES, it is also likely that the selection of the appropriate patients for DES implantation has improved over time. Furthermore, improved implantation techniques and the adoption of new stent types during this period of time may have contributed to a more favourable outcome.
Since the publication of the outcomes of the 2003-4 cohort, we have made a great effort in making the database more complete by extended merging with other registries for collection of missing data on baseline characteristics. Thus, in the recent publication we have been able to analyse a greater number of patients with complete information, with only 925 patients out of 47, 967 (1.9%) excluded from the analysis. We have also focused our analysis on a large number of patients receiving only one stent at the index revascularisation procedure (n=18, 953) thereby allowing us to be able to adjust for differences in lesion, vessel and stent characteristics in addition to clinical parameters.
Long-term follow-up on mortality
The most important finding in the 2003 -2004 patient cohort creating the «SCAAR scare» was the increased mortality in the long-term with diverging event curves for the two stent types. In the propensity score adjusted landmark analysis there was a 32% relative increase in the risk of death after the initial six months, corresponding to an absolute 0.5% increased mortality (Figure 1).
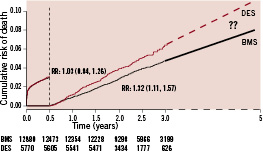
Figure 1. Landmark analysis of adjusted cumulative mortality at the mean level of the propensity score with drug eluting stents (dashed lines) or only with bare metal stents (solid lines) in all patients enrolled 2003-2004 and followed for 1-3 years. Modified from the N Engl J Med 2007;356:1009-19.
This finding was the reason for our conclusion that «a generalised, un-selective use of drug-eluting stents should be avoided until randomised studies with an adequate number of patients and long-term follow-up have ruled out any increased long-term risk.» When the follow-up of the cohort of patients enrolled 2003-2004 was extended to up to five years in the updated database, there was still a significant increase in late mortality. Reassuringly, there was no further divergence of the event curves and the adjusted risk ratio tended to be lower (Figure 2).
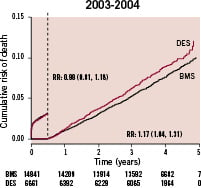
Figure 2. Landmark analysis of adjusted cumulative mortality at the mean level of the propensity score with drug eluting stents or only with bare metal stents in all patients enrolled 2003-2004 and followed for 3-5 years.
The larger number of patients with complete data included in this analysis did not impact the results in and of itself. When stratifying for year of inclusion the increased event rate was only seen in patients included 2003; i.e., when DES was introduced on the Swedish market. In contrast, when including all patients treated with one or more DES or BMS during the complete four year period (2003-2006) and followed for up to five years, the mortality was in fact lower in the first six months, and did not increased thereafter (Figure 3).
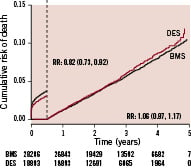
Figure 3. Landmark analysis of adjusted cumulative mortality at the mean level of the propensity score with drug eluting stents or only with bare metal stents in all patients enrolled 2003-2006 followed for 1-5 years.
In the cohort of patients treated with one stent at the index procedure, where it also was possible to adjust for differences in lesion and stent characteristics, the results were essentially the same, with a lower mortality the initial six months (OR 0.76; 95% C.I 0.64-91) with no significant difference thereafter (OR 1.08; 95% C.I 0.94-1.24).
When the SCAAR data were originally presented, they were considered to represent primarily off-label use of DES based on the approval of the U.S. Food and Drug Administration. However, in fact only half of the patients in SCAAR are treated according to DES off-label criteria, and there was no increase in adverse events with DES neither when used on- or off- label4.
Restenosis
During a four year follow-up of new angiography, restenosis was seen in 5.5% of BMS as compared to 4.5% DES. In a Cox regression analysis adjusting for differences in clinical, lesion and stent characteristics at baseline, the difference in restenosis rate between BMS and DES was greater (RR 0.43; 95% CI 0.36-0.52). A landmark analysis suggest that the whole reduction of restenosis with DES over BMS is achieved during the initial six months (R.R 0.34; 95% CI, 0.27-0.43) (Figure 4).
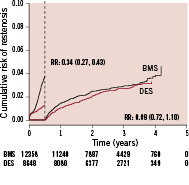
Figure 4. Landmark analysis of adjusted cumulative rate of restenosis for patients enrolled 2004-2006 for patients enrolled 2004-2006.
Thereafter, there was no significant difference between the stent types. The adjusted rates of restenosis for patients enrolled 2004, 2005 and 2006 showed a trend for lower risk ratios for DES vs. BMS over time (data not shown). Among many factors changing slightly over time, a continuous increase in average balloon pressure has been noted. This variable was added to the registry during the later period of the study. Thus, the mean maximum balloon pressure increased from 16.7± 2.8 Atm to 18.4±3.3 Atm from the third quarter 2006 to the last quarter 2008. Follow-up on 58,543 patients treated 2006-2008, showed that a higher balloon pressure was associated with a lower restenosis rate after multivariable adjustment for differences in background characteristics (Figure 5).
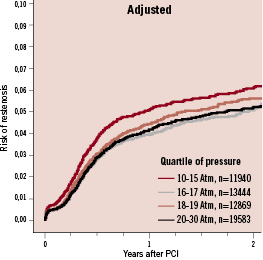
Figure 5. Adjusted cumulative rate of restenosis in relation to balloon pressure in all patients treated 2006-2008.
When we analysed rates of restenosis with different stent brands among the 62,822 implanted stents, we found considerable differences, with a range of restenosis from 4 to 12% after three years of follow-up5. After multivariable adjustment, including a propensity score, the cumulative probability of restenosis ranged from approximately 0.03 to 0.055 for DES and from 0.07 to 0.1 for BMS. Therefore, the absolute difference in restenosis rates between the most effective BMS and the least effective DES were larger than differences between the DES types as well as between the BMS types. In patients with diabetes, who are at high risk of restenosis, there are even larger differences between different DES types with regard to restenosis rates6. These results question the classification of DES (and BMS) as a class and reinforce that DES (as well as BMS) should be evaluated head-to-head in prospective randomised trials.
Stent thrombosis
Since March 2005, the database contains information about stent thrombosis defined as occlusion of a previously implanted stent with acute clinical presentation detected at any subsequent angiography. From March 2005 to December 2007, 65,066 stents were implanted at 41,470 procedures. Until February 2008, 747 cases of acute occlusions had been reported during a mean (SD) follow-up time of 564±276 days (SD)7. When stent implanted less than 400 times during the period where excluded, 61,513 stents remained.
When the stents were grouped into DES and BMS, different patterns regarding timing of stent thrombosis appeared between the two stent groups. The absolute rate of stent thrombosis was low, but after the initial six months, the rate was significantly increased with DES as compared to BMS and in fact twice as high at the end of follow up (Figure 6).
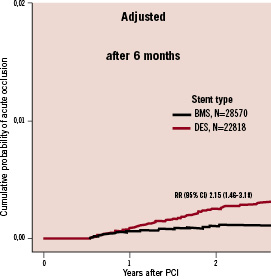
Figure 6. Landmark analysis of adjusted rate of acute stent occlusions after the initial six months in patients with one DES compared to one BMS 2005 to 2007.
These findings reinforce the need for randomised prospective comparisons between stent types, and emphasises the importance of the raised long-term risk of stent thrombosis after DES, although its clinical consequences over the years seems to have been reduced by a reduction in early restenosis and associated thrombosis and eventually also an increased use of long-term dual antiplatelet treatment.
In a retrospective evaluation of rates of stent thrombosis in different stent brands, we also found considerable differences with a range from 0.8 to 2% at 2.5 years. After multivariable adjustment, including a propensity score, the stent thrombosis cumulative probability ranged from approximately 0.03 to 0.05 for DES and from 0.07 to 0.1 for BMS. Our results again suggest that DES types have different properties and should be evaluated with head-to-head comparisons in prospective, randomised trials with long-term follow-up.
Drug eluting stent usage in Sweden
The average use of DES in Sweden increased steadily from 2002 and reached a peak in December 2005 before the reports of increased mortality at the European society of Cardiology meeting in September 2005 (Figure 7).
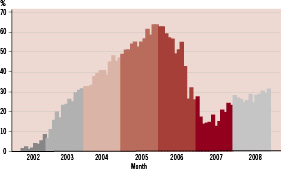
Figure 7. Average use of DES in Sweden per month 2002-2008.
After the initial publication of the SCAAR results in the New England Journal of Medicine, DES use has again slowly increased from approximately 10% to 30%. The SCAAR registry will continue to follow the overall DES utilisation rate and its effect on rates of restenosis, stent thrombosis, myocardial infarction and death, as well as the results of individual stent brands.
Discussion
The SCAAR registry has a unique opportunity to follow the experiences of established and new treatment modalities in a nationwide population without any patient selection and with complete follow-up on outcome variables. Given the fact that concealed confounders may influence the results when treatment options are studied, all registry data should primarily be considered hypothesis generating. However, in absence of adequate randomised trials, and for evaluation of trends and low frequent events, large scale registries such as the SCAAR are important for our understanding of new technologies and treatment options.
DES was associated with an increased event rate in patients treated in 2003 when these products became available on the Swedish market. For several probable reasons, among them an improved patient selection, implantation techniques and prolonged clopidogrel treatment, this outcome has improved over time. Long-term follow-up of the use of DES vs. BMS in the SCAAR registry now shows that use of DES overall is not associated with an increased mortality or raised risk of myocardial infarction as compared to BMS. In the SCAAR registry there is, however, still an increased long-term risk of stent thrombosis with DES. Although the risk for stent thrombosis on the average is low, it may be a very serious complication for the individual patient. Therefore, development of stents with lower risk profiles is warranted. An increased knowledge about the optimal duration, type and extent of platelet inhibition for the best balance of risk and benefit might also further reduce the overall risk. The ability to collect information on bleeding complications has recently been improved in the SCAAR registry and, by merging with the Swedish pharmacy registry, duration and dose of platelet inhibitors will be possible.
The absolute rate of restenosis currently is low as well as with DES as with BMS. Thus, a general use of DES in all-comers for PCI procedures has limited incremental gains, but large incremental costs, in comparison to BMS. The use of DES should, therefore, still be reserved for patients at risk of restenosis, such as diabetics and in long lesions with small diameters. In addition, DES use should be influenced by the patients long-term tolerance of dual anti-platelet treatment, because of the remaining problem of late stent thrombosis.

