
Abstract
Aims: The aim of this study was to assess the real-world clinical performance of a sirolimus-eluting ultrathin-strut drug-eluting stent (DES) (Orsiro) in a large nationwide cohort of patients undergoing percutaneous coronary intervention (PCI).
Methods and results: From the Swedish Coronary Angiography and Angioplasty Registry, the two-year outcomes of 4,561 patients implanted with Orsiro (Orsiro group) and 69,570 receiving other newer-generation DES (n-DES group) were analysed. The rate of definite stent thrombosis was low in both groups (0.67% and 0.83% for Orsiro and n-DES, respectively; adjusted hazard ratio [HR] 0.90, 95% confidence interval [CI]: 0.55-1.46, p-value 0.66). Restenosis was also infrequent (1.5% vs 2.0% with Orsiro and n-DES, adjusted HR 0.81, 95% CI: 0.63-1.03, p-value=0.09). The risk of target lesion revascularisation by PCI was lower in the Orsiro group (1.6% vs 2.3%, adjusted HR 0.75, 95% CI: 0.60-0.94, p-value=0.013). All-cause mortality and myocardial infarction did not show a statistically significant difference between the two groups (mortality of 7.5% in both groups, adjusted HR 0.99, 95% CI: 0.72-1.35, p-value=0.94; 6.0% vs 5.2% for myocardial infarction, adjusted HR 1.19, 95% CI: 1.00-1.43, p-value=0.06).
Conclusions: In a nationwide scenario, the use of a sirolimus-eluting ultrathin-strut DES portended favourable clinical outcomes.
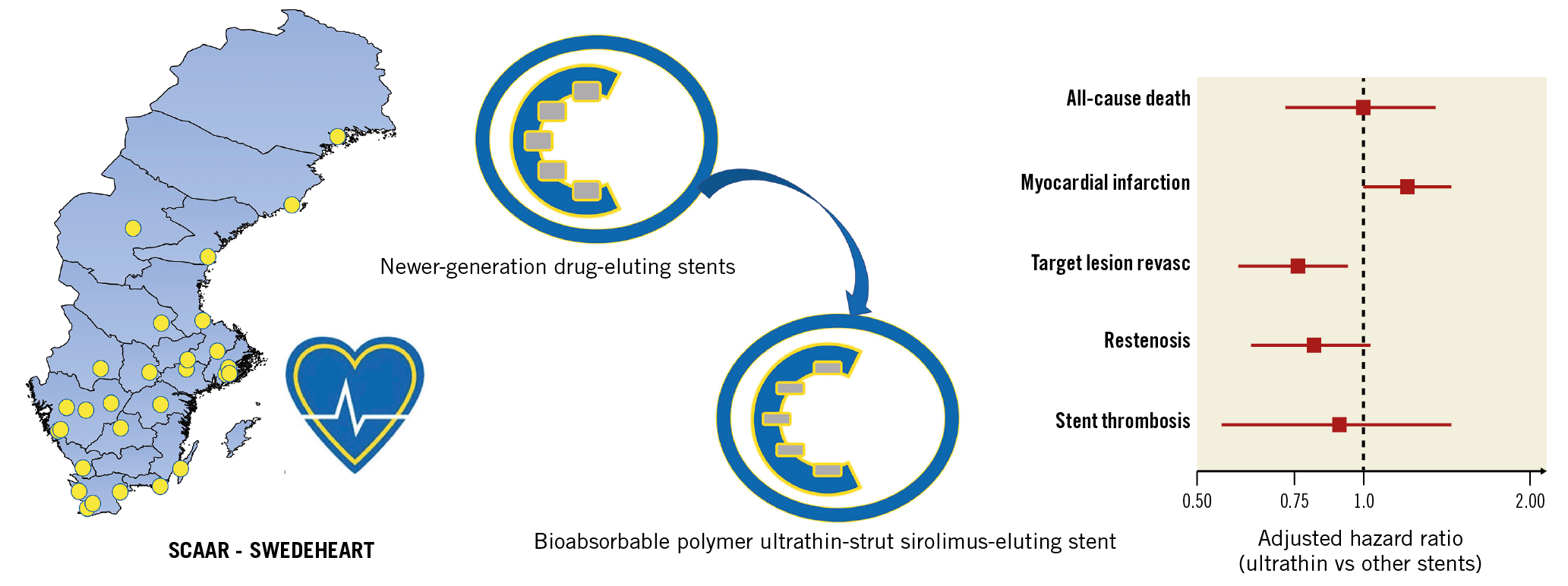
Visual summary. Clinical outcomes with unselected use of an ultrathin-strut sirolimus-eluting stent.
Introduction
Drug-eluting stents (DES) with ultrathin metallic platforms and preserved radial strength represent one of the latest advances in the field of contemporary DES technology with percutaneous coronary intervention (PCI)1. Recently, a meta-analysis of ten randomised clinical trials (RCTs) showed a significant reduction in the relative risk of target lesion failure at one year with the use of ultrathin DES platforms as compared to contemporary DES with relatively thicker stent struts2. It remains unclear whether in the real world PCI using this newer stent technology can provide incremental clinical benefits over the performance, already excellent, of other modern-generation DES. Indeed, RCTs have clear advantages when assessing the unbiased treatment effect of a new intervention, but at the same time they suffer from limitations due to stringent selection criteria and non-consecutive enrolment which may limit their generalisability3. Registries provide information on the efficacy and safety of a therapeutic strategy in consecutive and unselected cohorts of patients which is important and complementary to the results of RCTs4. Moreover, unique to large registries is the ability to assess and compare more thoroughly the incidence of low-frequency events such as stent thrombosis (ST).
To date, data regarding the clinical performance of ultrathin-strut DES in the real world are limited5,6. Therefore, we sought to assess the performance up to two years of a sirolimus-eluting ultrathin-strut DES (Orsiro; Biotronik AG, Bülach, Switzerland) in a large and unselected cohort of consecutive patients undergoing PCI in Sweden.
Methods
PATIENT POPULATION
All patients in this study were registered in the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) and details on registry design and performance have been reported previously7. Briefly, SCAAR is a prospective, multicentre registry which collects clinical data and procedural characteristics of all consecutive patients undergoing cardiac catheterisation in Sweden. The quality and reliability of information entered in the registry is periodically monitored against source clinical files (with levels of agreement above 97% in recent assessments)8. For this analysis, we selected patients who were implanted with only newer-generation DES (n-DES) from October 2011 (date of the first Orsiro implantation in Sweden) up to June 2017. To reflect general clinical practice more broadly, patients implanted with n-DES used in less than 1,000 implantations during the inclusion period were excluded. Also, to assess specifically the performance of the Orsiro stent, patients implanted with both Orsiro and other n-DES at the index procedure were excluded. Figure 1 shows the flow chart of the study population.
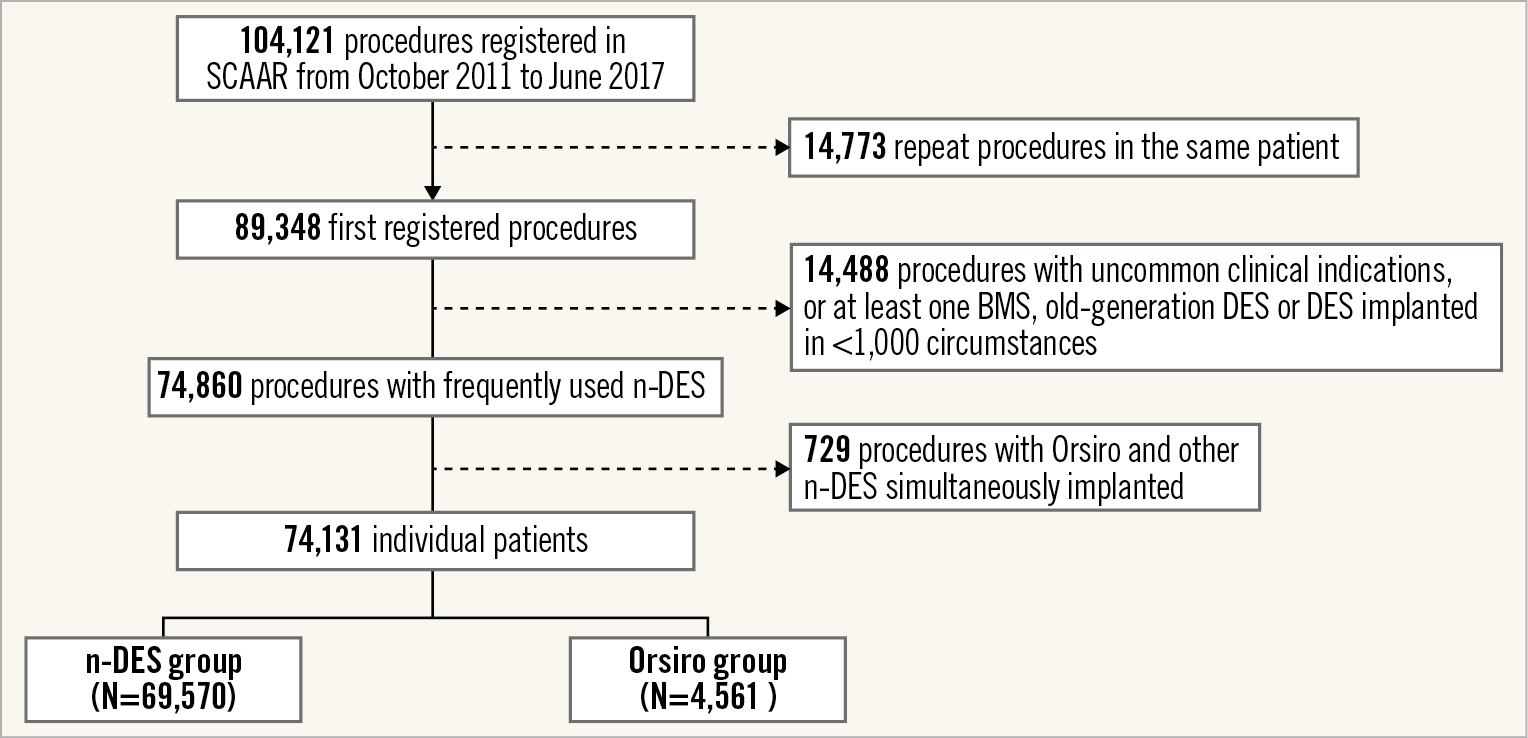
Figure 1. Flow diagram of the study population.
ORSIRO AND OTHER STENTS IN THIS STUDY
The structural characteristics of the Orsiro stent have been reported in detail elsewhere9. Orsiro is an ultrathin-strut (60 and 80 μm strut thickness for stent diameters ranging from 2.25 to 3.0 mm and from 3.5 to 4.0 mm, respectively), sirolimus-eluting, bioabsorbable polymer DES.
The following n-DES were included in this analysis and used as a control group, namely: XIENCE PRIME®, XIENCE Xpedition® and XIENCE ProX (Abbott Vascular, Santa Clara, CA, USA); PROMUS Element™, PROMUS Element™ Plus and Promus PREMIER™ (Boston Scientific, Marlborough, MA, USA); Resolute Integrity® and Resolute Onyx™ (Medtronic, Minneapolis, MN, USA); SYNERGY™ (Boston Scientific); Ultimaster® (Terumo Corporation, Tokyo, Japan).
After stent implantation, dual antiplatelet therapy was generally recommended for 6 or 12 months in patients undergoing PCI for stable coronary artery disease (CAD) or an acute coronary syndrome, respectively.
CLINICAL OUTCOMES AND DEFINITIONS
The principal outcomes of interest for this study were definite ST, clinically relevant restenosis, target lesion revascularisation (TLR) by PCI, myocardial infarction (MI) and all-cause mortality. Definitions of clinical outcomes assessed in this study are listed in Supplementary Appendix 1.
STATISTICAL ANALYSIS
Continuous and dichotomous parameters are reported as mean and standard deviation or as frequency and percentage, respectively. Differences in the clinical and procedural characteristics between patients implanted with Orsiro (Orsiro group) and other n-DES (n-DES group) were assessed using the standardised mean difference (SMD). SMD is a statistical measure of the effect size difference which is independent of the sample size. SMD values above 0.1 reflect the presence of potential imbalance between two groups10.
The cumulative incidence of events was assessed using the Kaplan-Meier method and the adjusted hazard ratio (HR) for the clinical outcomes of interest was calculated using weighted Cox proportional hazards regression models. Further details of the statistical analysis plan, including a number of sensitivity and subgroup analyses, are reported in Supplementary Appendix 2 and Supplementary Appendix 3.
Results
CLINICAL AND PROCEDURAL CHARACTERISTICS
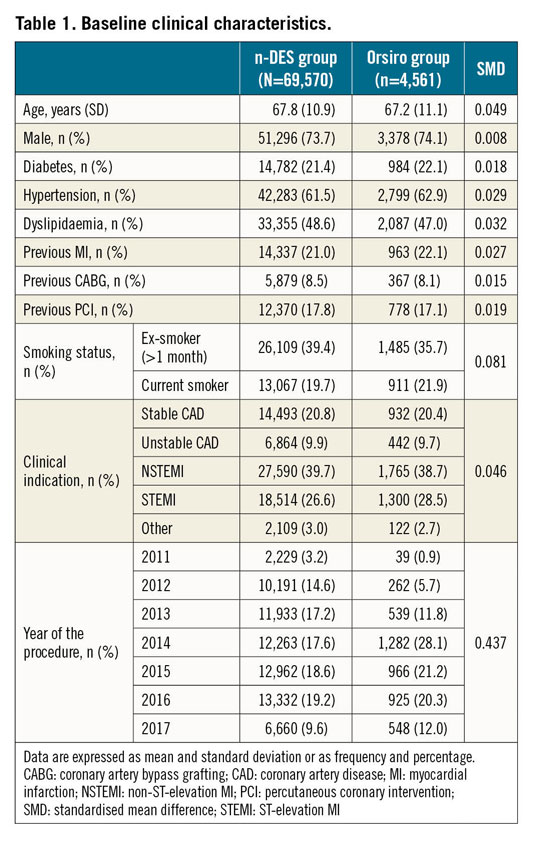
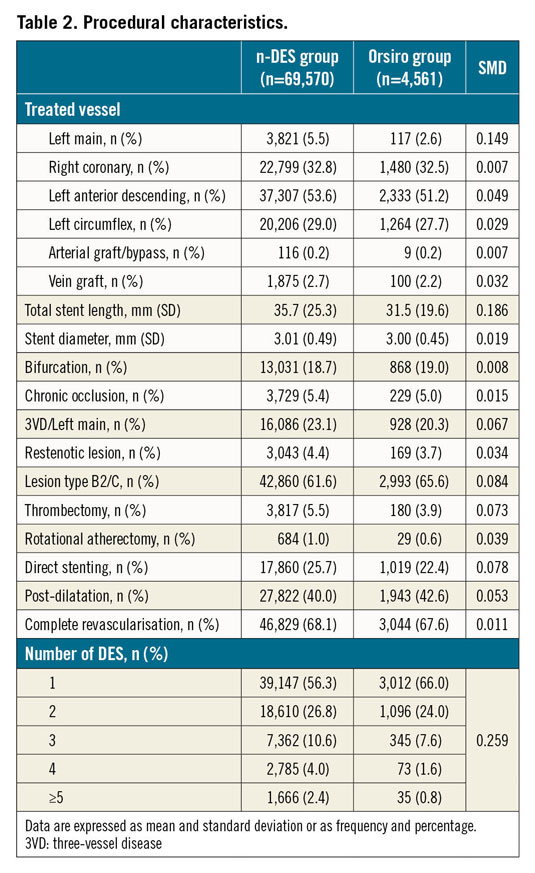
A total of 74,131 patients were included in the analysis. Of these, 4,561 patients were implanted with Orsiro (Orsiro group) at the index procedure and 69,570 with other n-DES (n-DES group). The baseline clinical and procedural characteristics of the study cohort are reported in Table 1 and Table 2, respectively. The use of Orsiro increased progressively during the inclusion period. There were no relevant differences between the two stent groups concerning the baseline clinical characteristics (all SMD values below 0.1). Of note, the majority of patients underwent PCI for an acute coronary syndrome (76.2% of patients). Regarding the procedural characteristics, Orsiro was less frequently implanted in patients undergoing left main PCI (2.6% vs 5.8%). The number of stents was higher and total stent length was longer at the index procedure in the n-DES as compared to the Orsiro group. The medications used before and during the index PCI procedure are presented in Supplementary Table 1.
WEIGHTING APPROACH AND ADJUSTED RISK ASSESSMENT
The distributional density of the propensity score (PS) in the two groups and the absolute SMD for all covariates included in the PS model (before and after weighting) are presented in Supplementary Figure 1. The weighting approach was effective at improving the overlap between the distributional density of the PS in the two groups, as well as reducing the SMD across all covariates included in the model (all SMD values below 0.1 after weighting). The effective sample size in the n-DES group after weighting was 54,893 patients.
CRUDE AND ADJUSTED OUTCOMES UP TO TWO YEARS
The Kaplan-Meier curves for the cumulative incidence of clinically relevant restenosis, definite ST and TLR by PCI are presented in Figure 2A-Figure 2C, respectively. The rate of ST was low in both stent groups (0.67% and 0.83% for Orsiro and other n-DES, respectively; adjusted HR 0.90, 95% confidence interval [CI]: 0.55-1.46, p-value 0.66). The timing of ST in the two groups is presented in Table 3. The rate of clinically relevant restenosis, albeit not significantly different, was numerically lower with Orsiro (1.5% vs 2.0%; adjusted HR 0.81, 95% CI: 0.63-1.03, p-value=0.09). Mirroring the reduced rates of ST and in-stent restenosis, the risk of TLR by PCI was lower in the group of patients treated with Orsiro (1.6% vs 2.3%, adjusted HR 0.75, 95% CI: 0.60-0.94, p-value=0.013). All-cause mortality, as shown in Figure 3A, was similar in the two groups (7.5% in both Orsiro and n-DES groups, adjusted HR 0.99, 95% CI: 0.72-1.35, p-value=0.94) while there was a numerically higher incidence of MI in the Orsiro group (Figure 3B) (6.0% vs 5.2%; adjusted HR 1.19, 95% CI: 1.00-1.43, p-value=0.06).
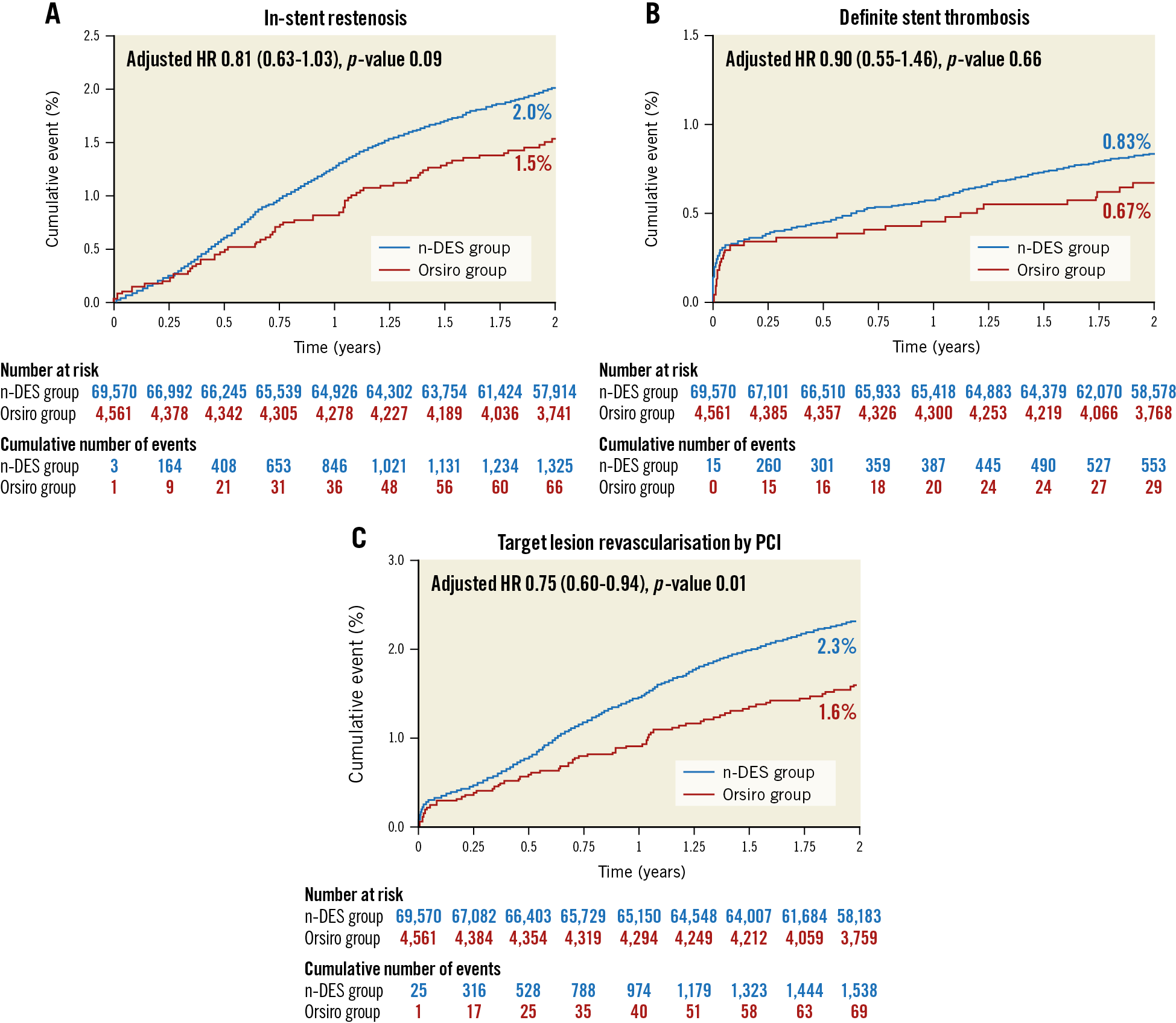
Figure 2. Clinical outcomes up to two years for clinically relevant in-stent restenosis (A), definite stent thrombosis (B) and target lesion revascularisation by percutaneous coronary intervention (C).
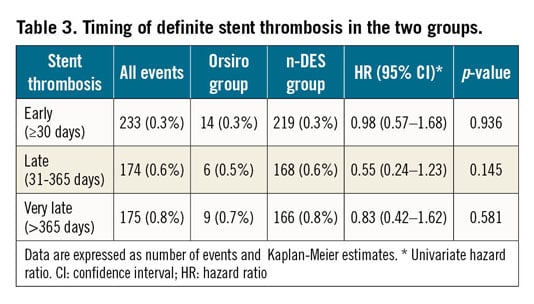
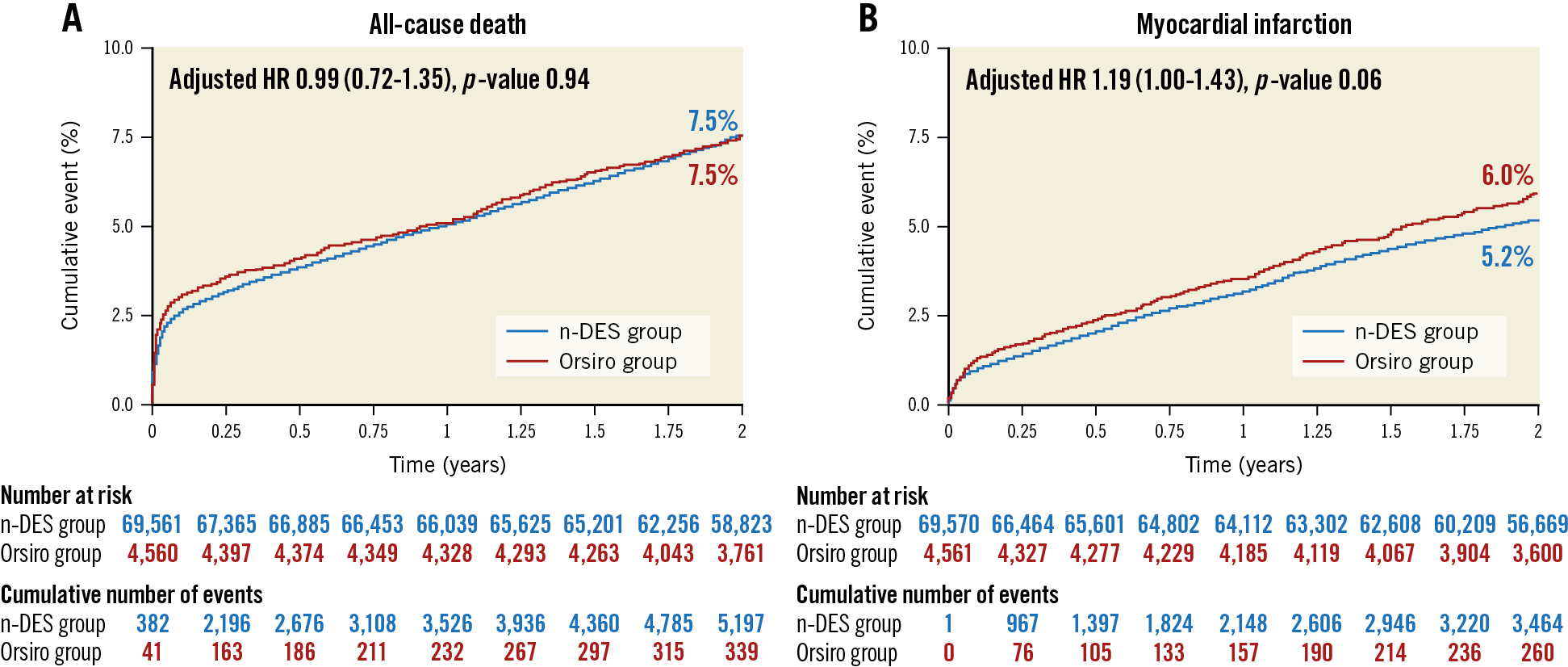
Figure 3. All-cause mortality (A) and acute myocardial infarction (B) up to two years.
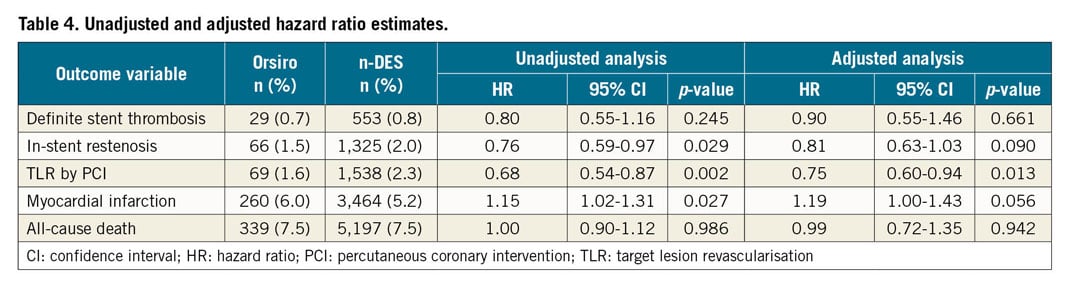
The unadjusted and adjusted HRs for all the outcomes of interest are reported in Table 4.
SENSITIVITY AND SUBGROUP ANALYSES
Results were consistent with the main analysis across several sensitivity analyses (Table 5). The subgroup analysis by clinical presentation (Figure 4) yielded consistent results among Orsiro and n-DES with respect to all clinical outcomes (all p-values for interaction >0.05). Stent-level outcomes (ST and restenosis) did not differ between stents having a diameter ≤ or >3.00 mm (Figure 5).
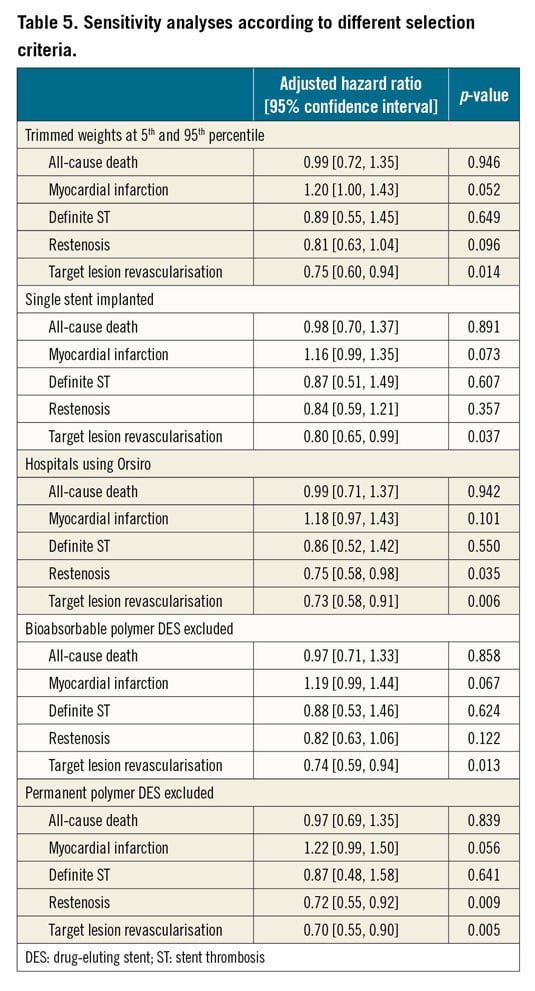
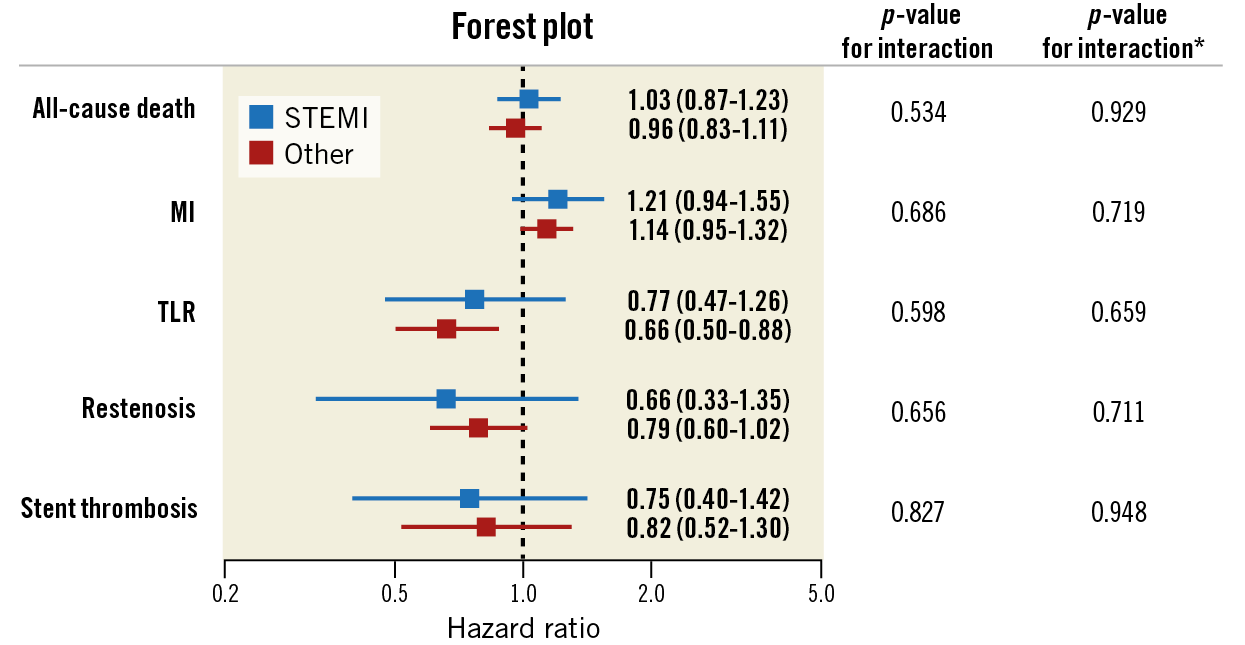
Figure 4. Subgroup analysis by clinical indication (ST-elevation myocardial infarction versus other clinical indications). Forest plot showing the univariate hazard ratios by clinical indication for the different clinical outcomes. * indicates interaction terms in the weighted models. MI: myocardial infarction; TLR: target lesion revascularisation
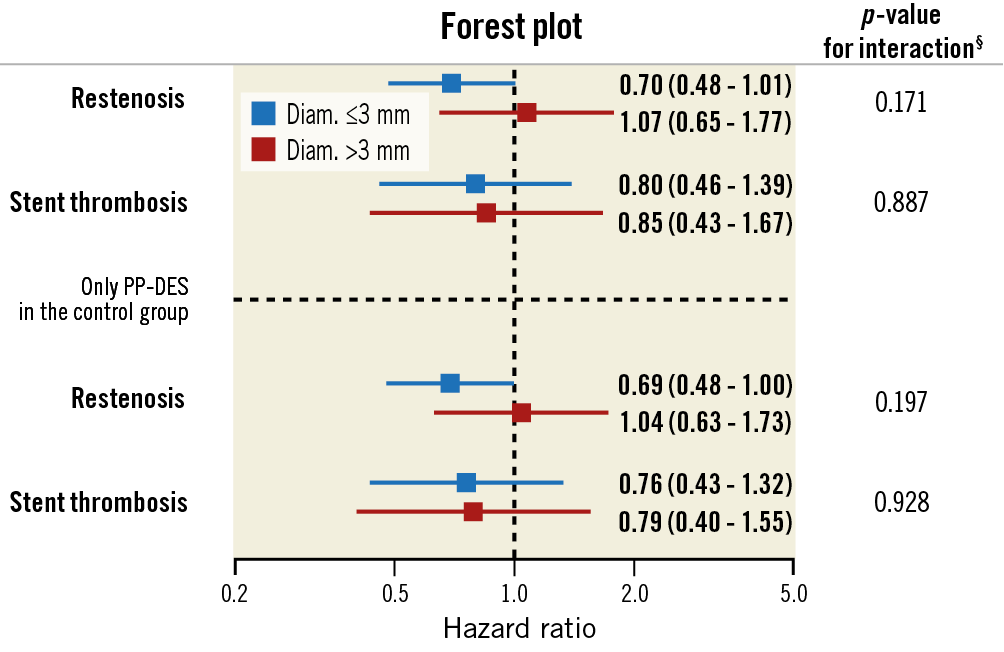
Figure 5. Subgroup analysis by stent diameter (stent-level analyses). Forest plot showing the univariate hazard ratios for restenosis and stent thrombosis. § interaction term by stent diameter accounting for clustering of multiple stents in the same patient.
Discussion
The principal findings of this study can be summarised as follows: a) the use of Orsiro, a sirolimus-eluting ultrathin-strut DES, is associated with favourable clinical outcomes up to two years in a large, real-life cohort of consecutive patients undergoing PCI; b) in adjusted analyses, Orsiro yielded a significantly lower risk of TLR by PCI as compared with other modern-generation DES; c) no differences in other clinical outcomes, including all-cause mortality and re-hospitalisation for MI, were seen between Orsiro and other n-DES; d) results were consistent across several sensitivity and subgroup analyses exploring the impact of more restrictive clinical and stent-related selection criteria. Of note, these results were observed in a cohort of patients with an acute coronary syndrome as the most frequent clinical indication for PCI.
A class effect for DES with ultrathin struts has recently been hypothesised2. Different mechanisms may explain the improved stent performance seen with ultrathin-strut DES. Stent strut thickness affects flow patterns and local shear stress inside the coronary arteries11. Thicker stent struts have been associated with disturbed coronary flow and non-uniform distribution of local shear stress. These factors are crucial regulators of the stent endothelialisation process and, when altered, lead to a hyper-proliferating and pro-atherogenic status in the affected endothelium12. Also, by preventing the shear stress-triggered release of growth factors from activated platelets, a lower strut thickness may be protective against exuberant endothelial proliferation13.
Corroborating the emerging evidence of improved stent performance with the use of ultrathin-strut DES, this real-world analysis demonstrated lower rates of stent failure (numerically lower rates of ST and restenosis, lower risk of TLR by PCI) with Orsiro as compared with other newer-generation DES frequently used in Sweden. Our findings are consistent with the two-year results of the Biotronik Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment of Subjects with Up to Three De Novo or Restenotic Coronary Artery Lesions V (BIOFLOW-V) study showing a significantly lower risk of TLR with Orsiro as compared with XIENCE14. Also, a landmark analysis between one and two years of the Comparison of Biodegradable Polymer and Durable Polymer Drug-eluting Stents in an All Comers Population (BIO-RESORT) trial demonstrated a significant reduction of TLR with Orsiro as compared with Resolute Integrity (zotarolimus-eluting DES)15. It must be acknowledged that registries have inherent limitations when looking at the comparative performance of different interventions. Indeed, allocation bias and residual confounding may represent important sources of bias in the estimation of the (adjusted) treatment effects of an intervention. As an example, lower total stent length implanted in patients treated with Orsiro in our analysis, alongside the more frequent implantation of a single stent in the Orsiro group, might reflect a higher likelihood of Orsiro implantation in less complex anatomical lesion subsets. We tried to minimise the risk of confounding in our analysis by including both total stent length and the number of implanted stents in the PS model. In addition, lesion complexity as evaluated by the American Heart Association/American College of Cardiology grading system and the use of rotational atherectomy (as a marker of severe calcification) were also incorporated in the PS model. Finally, the results were consistent in a sensitivity analysis restricted to patients implanted with a single stent at the index procedure.
The presence of a bioabsorbable polymer with enhanced biocompatibility in the Orsiro stent could also represent a potential mechanism associated with improved stent performance. Engineering a polymer to disappear within a specific time window has been advocated as a potential mechanism to improve the long-term efficacy of modern DES. However, bioabsorbable polymer DES have been demonstrated to be at least non-inferior in several RCTs and, to date, no signs of a sustained clinical benefit with the use of this technology have clearly emerged16,17. Moreover, resorption of the poly-L-lactic acid polymer in the Orsiro stent occurs up to two years18. This implies that the benefits attributable to the absence of polymer are limited in the current analysis which is not extended beyond the time of complete polymer resorption.
Finally, in the BIOFLOW-V study, the difference in target vessel failure favouring Orsiro was driven by a significant reduction in the risk of target vessel-related MI18. A landmark analysis at 30 days demonstrated that the reduction in MI occurred both in the periprocedural setting and in the longer term after the procedure14. The potential benefits in reducing the risk of MI with Orsiro have also been confirmed in a more comprehensive meta-analysis of six RCTs19. MI risk did not differ between Orsiro and other modern-generation DES in this registry-based analysis. Some limitations in the definition of MI in this study largely account for these seemingly inconsistent results. Indeed, MI definition was based entirely on administrative data (ICD codes) related to new hospitalisations for MI in patients presenting with elevated cardiac biomarkers. However, serial biomarker assessments also during the index hospitalisation are essential for diagnosing periprocedural MI. Also, the evaluation of MI with ICD codes did not allow a vessel-oriented classification of new MI cases. This aspect is important since it is known that recurrent MIs are more likely to arise from the progression of previously unstented lesions20.
Limitations
Beside the definition of MI and the risk of residual confounding in registry-based analyses, this study has additional limitations. The definition of TLR did not include cases of repeat revascularisation by coronary artery bypass grafting and, although ICD codes for MI are regularly monitored in RIKS-HIA, we did not adjudicate individual MI cases. The selection of patients who were implanted only with Orsiro may have introduced selection bias into this study. Finally, SCAAR does not collect data on adherence over time to the prescribed treatment with antiplatelet agents and other medications used for secondary cardiovascular prevention. Similarly, information on patients who were treated with prolonged dual antiplatelet therapy was not available in the registry.
Conclusions
In a large nationwide cohort of patients undergoing PCI, the use of an ultrathin-strut sirolimus-eluting stent portended favourable clinical outcomes. These findings complement current evidence from RCTs and may help to support the decision-making process regarding the selection and use of modern DES.
|
Impact on daily practice In randomised clinical trials, drug-eluting stents with ultrathin metallic platforms have been demonstrated to reduce the rate of stent failure and improve clinical outcomes. By looking at the comparative performance of modern DES in a nationwide scenario, this study confirms the favourable clinical performance of an ultrathin-strut sirolimus-eluting stent. The finding of a numerical excess of MI events in the group of patients who received an ultrathin-strut sirolimus-eluting stent is not consistent with the results of randomised clinical trials; unmeasured confounders (i.e., DAPT duration), different MI definitions or the play of chance may partly account for these divergent findings. |
Funding
This study was financially supported by Biotronik with an institutional research grant. The sponsor did not influence the collection, analysis or interpretation of the data. The SCAAR registry is funded solely by the Swedish government and the Swedish Association of Local Authorities and Regions.
Conflict of interest statement
S. Buccheri received a research grant from the European Society of Cardiology during the conduct of this study. O. Fröbert reports personal fees from Biotronik (<5,000 USD). S. James has received institutional research grants from Abbott Vascular, Biotronik and Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

