Abstract
Background: Target lesion failure (TLF) remains an issue with contemporary drug-eluting stents. The dual-therapy sirolimus-eluting and CD34 antibody-coated COMBO stent (DTS) was designed to improve early healing.
Aims: We aimed to compare the 3-year outcomes of the DTS and the sirolimus-eluting Orsiro stent (SES) in all-comer patients treated with percutaneous coronary intervention.
Methods: The SORT OUT X trial is a prospective multicentre randomised clinical trial with a registry-based follow-up comparing DTS and SES. The primary endpoint, TLF, is a composite of cardiac death, myocardial infarction or target lesion revascularisation (TLR).
Results: A total of 3,146 patients were randomised to treatment with the DTS (1,578 patients) or the SES (1,568 patients). At 3 years, an intention-to-treat analysis showed that 155 patients (9.8%) who were assigned the DTS and 118 patients (7.5%) who were assigned the SES met the primary endpoint (incidence rate ratio for TLF=1.33, 95% confidence interval: 1.04-1.70; p=0.02). This difference was caused by a significantly higher TLF rate in the DTS group compared to the SES group within the first year, which was mainly explained by a higher incidence of TLR in the DTS group compared to the SES group. Of note, the TLF rates were almost identical from 1 year to 3 years in both stent groups.
Conclusions: At 3 years, the SES was superior to the DTS, mainly because the DTS was associated with an increased risk of TLF within the first year but not from 1 to 3 years. ClinicalTrials.gov: NCT03216733.
Introduction
Coronary events, including stent thrombosis and in-stent restenosis, remain an issue with contemporary drug-eluting stents (DES). Therefore, attempts have been made to improve early healing with neointimal stent strut coverage. The dual-therapy sirolimus-eluting COMBO stent (DTS; OrbusNeich) combines an abluminal, bioabsorbable polymer with a luminal CD34 antibody designed to capture endothelial progenitor cells. In a porcine study, the DTS showed promising results with less neointimal thickness and a higher degree of endothelial cell adhesion molecule expression compared to a sirolimus-eluting stent or an everolimus-eluting stent. Thus, the DTS appeared to promote endothelialisation while reducing neointimal formation and inflammation1. The DTS has been compared to first- and second-generation DES in 3 smaller randomised controlled trials234. In these studies, the DTS was found to be non-inferior. A recent study evaluated the performance of the DTS in a large contemporary cohort of patients using patient-level data and concluded that the low rates of primary and secondary endpoints suggest that DTS may be a good alternative to other contemporary DES5. The SORT OUT X trial presented the first head-to-head comparison of the DTS to the third-generation sirolimus-eluting Orsiro stent (SES; BIOTRONIK)6. The SES was found to be superior to the DTS after 12 months, mainly because the DTS was associated with an increased risk of target lesion revascularisation (TLR). Herein, we report the prespecified analysis of the 3-year clinical outcomes of the SORT OUT X trial.
Methods
Patients and study design
SORT OUT X was a randomised, multicentre, single-blind, all-comer, two-arm, blinded-endpoint, non-inferiority trial, comparing DTS to SES in all types of coronary artery lesions. The trial was carried out at 3 large university hospitals in Western Denmark (Aarhus, Aalborg and Odense). Patients were eligible if they were at least 18 years old and had coronary artery disease requiring treatment with a DES. If multiple lesions were treated, the allocated study stent had to be used in all lesions. Exclusion criteria were allergy to aspirin, clopidogrel, ticagrelor, prasugrel, or sirolimus; participation in another randomised stent trial; inability to provide written informed consent; or life expectancy of less than 1 year. The study complied with the Declaration of Helsinki and was approved by the Regional Committees on Health Research Ethics for Central Denmark Region (1-10-72-38-17) and the Danish Data Protection Agency (1-16-02-14-17). All patients provided informed consent for trial participation before randomisation. Randomisation, study stents and use of antithrombotic medication are described in the primary publication6.
Outcome measures
Definitions of the endpoints were provided in the main publication6. The primary endpoint, target lesion failure (TLF), is a composite of cardiac death, myocardial infarction (MI) not related to other than index lesion, or TLR with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) within 3 years. Individual components of the primary endpoint comprise the secondary endpoints: MI; clinically indicated TLR; death (cardiac and non-cardiac); and target vessel revascularisation (TVR); as well as definite, probable, possible, and overall stent thrombosis according to the Academic Research Consortium definition7; and a patient-related composite endpoint (all death, all MI, or any revascularisation).
Clinical event detection
The study was based on clinically driven event detection, and no dedicated follow-up was scheduled. At 3-year follow-up, data on mortality, hospital admission, coronary angiography, repeat PCI, and CABG were obtained from the following national Danish administrative and healthcare registries: the Civil Registration System8; the Western Denmark Heart Registry9; and the Danish National Registry of Patients10. The latter maintains records on all hospitalisations in Denmark. The National Health Service provides tax-supported healthcare, guaranteeing unfettered access to medical care. All acute medical conditions are exclusively treated at public hospitals in Denmark. The Danish Civil Registration System has kept electronic records on sex, birth date, residence, emigration date, and vital status changes since 1968, with daily updates; the 10-digit civil registration number assigned at birth and used in all registries allows accurate record linkage. Loss to follow-up was minimised in the study, as vital status data for our study participants were provided by the Civil Registration System. The Danish National Registry of Patients provided information on diagnoses assigned by the treating physician during hospitalisations (coded according to the International Classification of Diseases, 10th revision [ICD-10])11.
An independent event committee reviewed all endpoints and source documents to adjudicate causes of death, reasons for hospital admission, and diagnosis of MI. Two dedicated PCI operators at each participating centre reviewed cine films for the event committee to classify stent thrombosis, TLR, and TVR (with either PCI or CABG). The independent event committee was blinded to study stent type assignment during the adjudication process. This methodology has been used in the previous SORT OUT studies121314.
Statistical analysis
Distributions of continuous variables between study groups were compared using the 2-sample Student’s t-test (or the Cochran test for cases of unequal variance) or the Mann-Whitney U test, depending on whether the data followed a normal distribution. Distributions of categorical variables were analysed using the chi-square test. In analyses of every endpoint, follow-up continued until the date of an endpoint event, death, emigration, or 3 years after stent implantation, whichever came first. Cumulative incidence curves were constructed based on time to events, accounting for the competing risk of death. The reference group comprised the patients randomised to the SES stent for overall and subgroup analyses. Incidence rate ratios (IRR) were calculated for TLF at 3-year follow-up and for prespecified patient subgroups (based on baseline demographic and clinical characteristics). In all analyses, the intention-to-treat principle was used. A 2-sided p-value of less than 0.05 indicated statistical significance. Analyses were performed on a patient level. SAS software, version 9.4 (SAS Institute) was used for the analyses. This trial is registered at ClinicalTrials.gov: NCT03216733.
Results
Between June 2017 and December 2018, 3,146 patients were randomly assigned to receive either the DTS (1,578 patients) or the SES (1,568 patients). Six patients were lost to follow-up due to emigration (total follow-up: 99.8%). Baseline patient characteristics (Table 1) and selected lesion and procedural characteristics (Table 2) did not differ significantly between the 2 stent groups. The mean age was 66.9±10.8 years, diabetes mellitus was present in 17.5% of the patients, and a high proportion of patients in both groups had acute coronary syndromes (54%), multivessel disease (25%), and complex lesions (B2 or C, 78%) (Table 1, Table 2). At 3 years, the composite endpoint, TLF, occurred in 155 patients (9.8%) in the DTS group and in 118 patients (7.5%) in the SES group (IRR for TLF=1.33, 95% confidence interval [CI]: 1.04-1.70; p=0.02) (Figure 1, Table 3). This difference was caused by a significantly higher TLF rate in the DTS group compared to the SES group within the first year; of note, the TLF rates were almost identical from 1 year to 3 years in both stent groups. The cumulative incidences of death, cardiac death, and MI were comparable up to 3 years between the 2 stent groups (Figure 1), and the rates of these endpoints at 3 years did not differ significantly (Table 3). However, the incidence proportion and the rates of TLR were higher among the DTS-treated patients (n=78 [4.9%]) compared with the SES-treated patients (n=55 [3.5%]; IRR=1.44, 95% CI: 1.01-2.03; p=0.04) (Figure 1, Table 3). Again, this difference was caused by a significantly higher TLR rate in the DTS group compared to the SES group within the first year, while the TLR rates did not differ significantly from 1 year to 3 years between the 2 stents. The cumulative incidence of definite stent thrombosis up to 3 years showed a comparable pattern in the 2 groups, and rates of definite stent thrombosis at 3 years were not different between the 2 groups (Figure 1, Table 3). Findings for the primary endpoint, TLF, were consistent across prespecified subgroups (Figure 2).
Table 1. Baseline characteristics of the study population.
| DTS (N=1,578) | SES (N=1,568) | p-value | ||
|---|---|---|---|---|
| Age, mean (SD), years | 67.1 (10.7) | 66.7 (10.9) | 0.32 | |
| Men, n (%) | 1,213 (76.9) | 1,208 (77.0) | 0.91 | |
| Diabetes mellitus, n (%) | 279 (17.7) | 271 (17.3) | 0.77 | |
| Arterial hypertension, n (%) | 835 (53.7) | 871 (56.6) | 0.11 | |
| Hypercholesterolaemia, n (%) | 783 (50.3) | 783 (50.7) | 0.81 | |
| Current smoker, n (%) | 410 (29.1) | 429 (30.5) | 0.42 | |
| Body mass index, mean (SD), kg/m2 | 28.0 (4.8) | 27.9 (4.7) | 0.48 | |
| Previous myocardial infarction, n (%) | 240 (15.4) | 221 (14.5) | 0.48 | |
| Previous percutaneous coronary intervention, n (%) | 295 (18.9) | 303 (19.7) | 0.58 | |
| Previous coronary artery bypass grafting, n (%) | 111 (7.1) | 89 (5.8) | 0.13 | |
| Indication for percutaneous coronary intervention | ST-segment elevation myocardial infarction, n (%) | 389 (24.7) | 355 (22.6) | 0.32 |
| Non-ST-segment elevation myocardial infarction or unstable angina, n (%) | 467 (29.6) | 499 (31.8) | ||
| Stable angina, n (%) | 651 (41.3) | 654 (41.7) | ||
| Other, n (%) | 71 (4.5) | 60 (3.8) | ||
| Comorbidity index score | 0, n (%) | 844 (53.5) | 849 (54.2) | 0.37 |
| 1-2, n (%) | 555 (35.2) | 521 (33.2) | ||
| 3+, n (%) | 179 (11.3) | 198 (12.6) | ||
| Differences between stents were tested by ꭓ2 statistics in categorical variables presented as numbers (n) and proportions (%), by the t-test in continuous variables presented as mean and standard deviation (SD). DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; IQR: interquartile range; SD: standard deviation; SES: sirolimus-eluting stent | ||||
Table 2. Baseline lesion and procedural characteristics and treatment.
| DTS (N=1,578) | SES (N=1,568) | p-value | ||
|---|---|---|---|---|
| Number of lesions | 2,008 | 1,982 | ||
| Target lesions per patient | 1, n (%) | 1,172 (74.3) | 1,175 (74.9) | 0.46 |
| 2, n (%) | 321 (20.3) | 312 (19.9) | ||
| 3, n (%) | 70 (4.4) | 57 (3.6) | ||
| >3, n (%) | 14 (0.9) | 23 (1.5) | ||
| No. per patient, n (%) | 1 (0.1) | 1 (0.1) | ||
| Target vessel location | Left main artery, n (%) | 54 (2.7) | 50 (2.5) | 0.22 |
| Left anterior descending artery, n (%) | 859 (42.8) | 905 (45.7) | ||
| Left circumflex artery, n (%) | 439 (21.9) | 440 (22.2) | ||
| Right artery, n (%) | 639 (31.8) | 567 (28.6) | ||
| Saphenous vein graft, n (%) | 16 (0.8) | 19 (1.0) | ||
| Lesion type | A, n (%) | 184 (9.2) | 210 (10.6) | 0.28 |
| B1, n (%) | 567 (28.3) | 585 (29.5) | ||
| B2, n (%) | 431 (21.5) | 403 (20.3) | ||
| C, n (%) | 825 (41.1) | 783 (39.5) | ||
| Chronic total occlusion lesions, n (%) | 89 (4.4) | 103 (5.2) | 0.26 | |
| Bifurcation lesions, n (%) | 481 (24.0) | 451 (22.8) | 0.37 | |
| Lesion length, mean (SD), mm | 22.8 (15.6) | 22.8 (15.8) | 0.95 | |
| Reference vessel size, mean (SD), mm | 3.4 (0.6) | 3.4 (0.6) | 0.68 | |
| No. of stents per patient, mean (SD) | 1.7 (1.0) | 1.7 (1.1) | 0.79 | |
| IVUS used, n (%) | 47 (2.3) | 56 (2.8) | 0.33 | |
| OCT used, n (%) | 10 (0.5) | 11 (0.6) | 0.80 | |
| Rotational atherectomy used, n (%) | 6 (0.3) | 13 (0.7) | 0.10 | |
| Differences between stents were tested by ꭓ2 statistics in categorical variables presented as numbers (n) and proportions (%), and by the t-test in continuous variables presented as mean and standard deviation (SD). DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; IQR: interquartile range; IVUS: intravascular ultrasound; OCT: optical coherence tomography; SD: standard deviation; SES: sirolimus-eluting stent | ||||
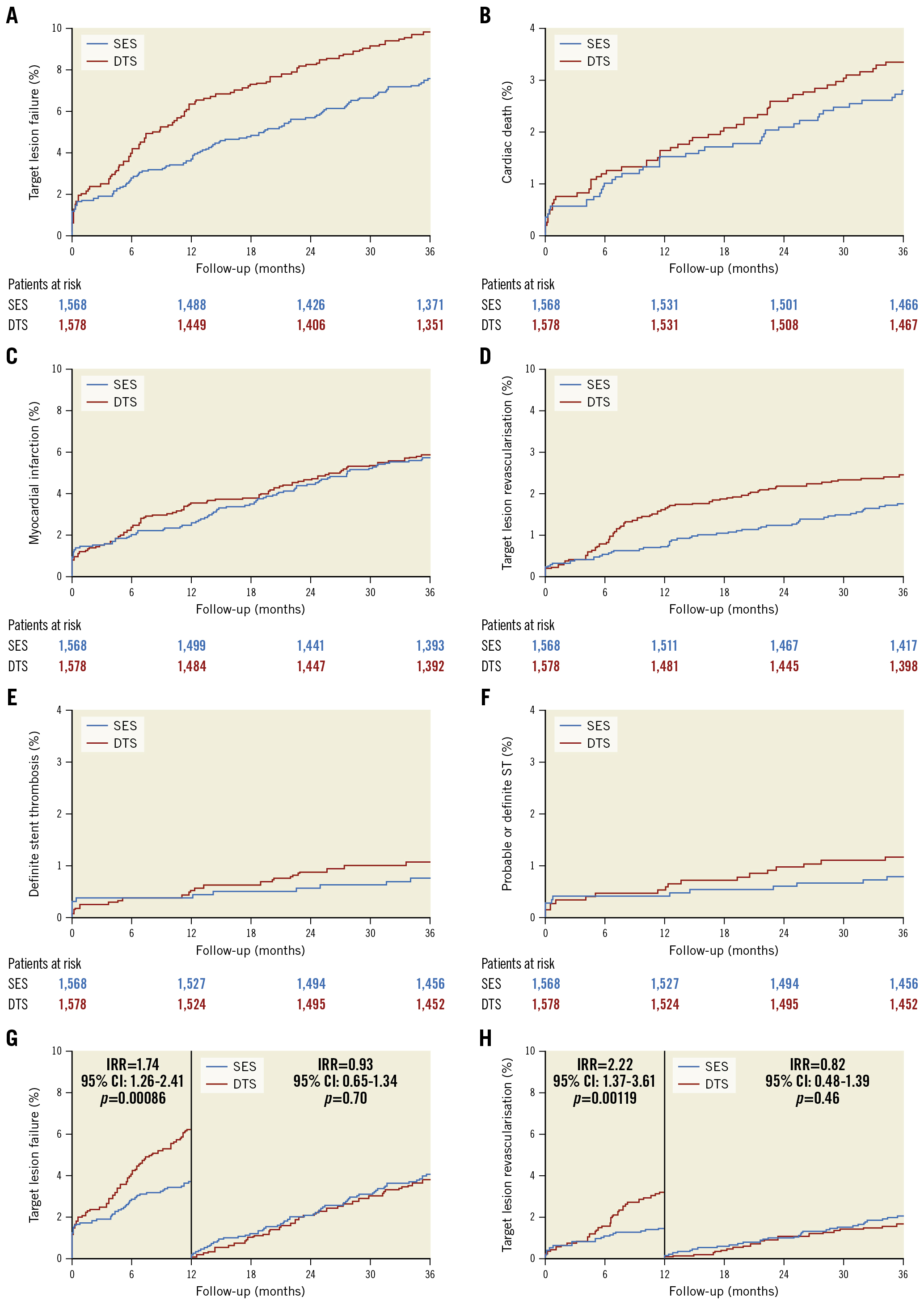
Figure 1. Time-to-event curves for major adverse cardiac events. A) Target lesion failure, B) cardiac death, C) myocardial infarction, D) target lesion revascularisation, E) definite stent thrombosis, F) probable or definite stent thrombosis, G) target lesion failure with landmark analysis, H) target lesion revascularisation with landmark analysis. CI: confidence interval; DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; IRR: incidence rate ratio; SES: sirolimus eluting stent; ST: stent thrombosis
Table 3. Three-year clinical outcomes.
| Outcome | DTS n=1,578 |
SES n=1,568 |
Incidence rate ratio (95% CI) |
p-value | |
|---|---|---|---|---|---|
| Target lesion failure | 0-3 years | 155 (9.8) | 118 (7.5) | 1.33 (1.04-1.70) | 0.02 |
| 0-1 years | 100 (6.3) | 58 (3.7) | 1.74 (1.26-2.41) | 0.00086 | |
| 1-3 years | 55 (3.8) | 60 (4.0) | 0.93 (0.65-1.34) | 0.70 | |
| Death | |||||
| All-cause mortality | 0-3 years | 108 (6.8) | 100 (6.4) | 1.08 (0.82-1.41) | 0.59 |
| 0-1 years | 46 (2.9) | 35 (2.2) | 1.31 (0.84-2.04) | 0.23 | |
| 1-3 years | 62 (4.0) | 65 (4.2) | 0.95 (0.67-1.35) | 0.78 | |
| Cardiac death | 0-3 years | 53 (3.4) | 44 (2.8) | 1.20 (0.80-1.79) | 0.37 |
| 0-1 years | 26 (1.6) | 24 (1.5) | 1.08 (0.62-1.89) | 0.78 | |
| 1-3 years | 27 (1.8) | 20 (1.3) | 1.35 (0.76-2.40) | 0.31 | |
| Non-cardiac death | 0-3 years | 55 (3.5) | 56 (3.6) | 0.98 (0.68-1.42) | 0.91 |
| 0-1 years | 20 (1.3) | 11 (0.7) | 1.81 (0.87-3.79) | 0.11 | |
| 1-3 years | 35 (2.3) | 45 (2.9) | 0.78 (0.50-1.21) | 0.26 | |
| MI (target-lesion related) | 0-3 years | 65 (4.1) | 59 (3.8) | 1.10 (0.77-1.57) | 0.59 |
| 0-1 years | 43 (2.7) | 29 (1.8) | 1.49 (0.93-2.38) | 0.10 | |
| 1-3 years | 22 (1.5) | 30 (2.0) | 0.73 (0.42-1.27) | 0.27 | |
| MI | 0-3 years | 93 (5.9) | 90 (5.7) | 1.03 (0.77-1.38) | 0.83 |
| 0-1 years | 55 (3.5) | 39 (2.5) | 1.41 (0.93-2.13) | 0.10 | |
| 1-3 years | 38 (2.6) | 51 (3.4) | 0.74 (0.49-1.13) | 0.17 | |
| Stent thrombosis | |||||
| Definite | 0-3 years | 17 (1.1) | 12 (0.8) | 1.42 (0.68-2.97) | 0.36 |
| 0-1 years | 8 (0.5) | 6 (0.4) | 1.33 (0.46-3.84) | 0.60 | |
| 1-3 years | 9 (0.6) | 6 (0.4) | 1.50 (0.53-4.22) | 0.44 | |
| Probable | 0-3 years | 2 (0.1) | 1 (0.1) | 2.00 (0.18-22.0) | 0.57 |
| 0-1 years | 2 (0.1) | 1 (0.1) | 2.00 (0.18-22.0) | 0.57 | |
| 1-3 years | 0 (0.0) | 0 (0.0) | |||
| Definite or probable | 0-3 years | 19 (1.2) | 13 (0.8) | 1.46 (0.72-2.96) | 0.29 |
| 0-1 years | 10 (0.6) | 7 (0.4) | 1.43 (0.54-3.75) | 0.47 | |
| 1-3 years | 9 (0.6) | 6 (0.4) | 1.50 (0.53-4.22) | 0.44 | |
| Target vessel revascularisation | 0-3 years | 113 (7.2) | 89 (5.7) | 1.29 (0.97-1.71) | 0.076 |
| 0-1 years | 80 (5.1) | 44 (2.8) | 1.84 (1.27-2.66) | 0.0013 | |
| 1-3 years | 33 (2.3) | 45 (3.0) | 0.75 (0.48-1.17) | 0.20 | |
| Target lesion revascularisation | 0-3 years | 78 (4.9) | 55 (3.5) | 1.44 (1.01-2.03) | 0.041 |
| 0-1 years | 53 (3.4) | 24 (1.5) | 2.22 (1.37-3.61) | 0.0012 | |
| 1-3 years | 25 (1.7) | 31 (2.1) | 0.82 (0.48-1.39) | 0.46 | |
| Patient-related endpoint | 0-3 years | 344 (21.8) | 312 (19.9) | 1.12 (0.95-1.31) | 0.17 |
| 0-1 years | 235 (14.9) | 186 (11.9) | 1.28 (1.05-1.56) | 0.016 | |
| 1-3 years | 109 (8.1) | 126 (9.1) | 0.88 (0.68-1.14) | 0.33 | |
| Values are n (%). The cumulative incidence of a particular event in the given period was calculated with death as a competing risk. The patient-related endpoint included all death, all myocardial infarctions, or any revascularisation. Target lesion failure included cardiac death, target vessel myocardial infarctions, or ischaemia-driven target lesion revascularisation. CI: confidence interval; DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; MI: myocardial infarction; SES: sirolimus-eluting stent | |||||
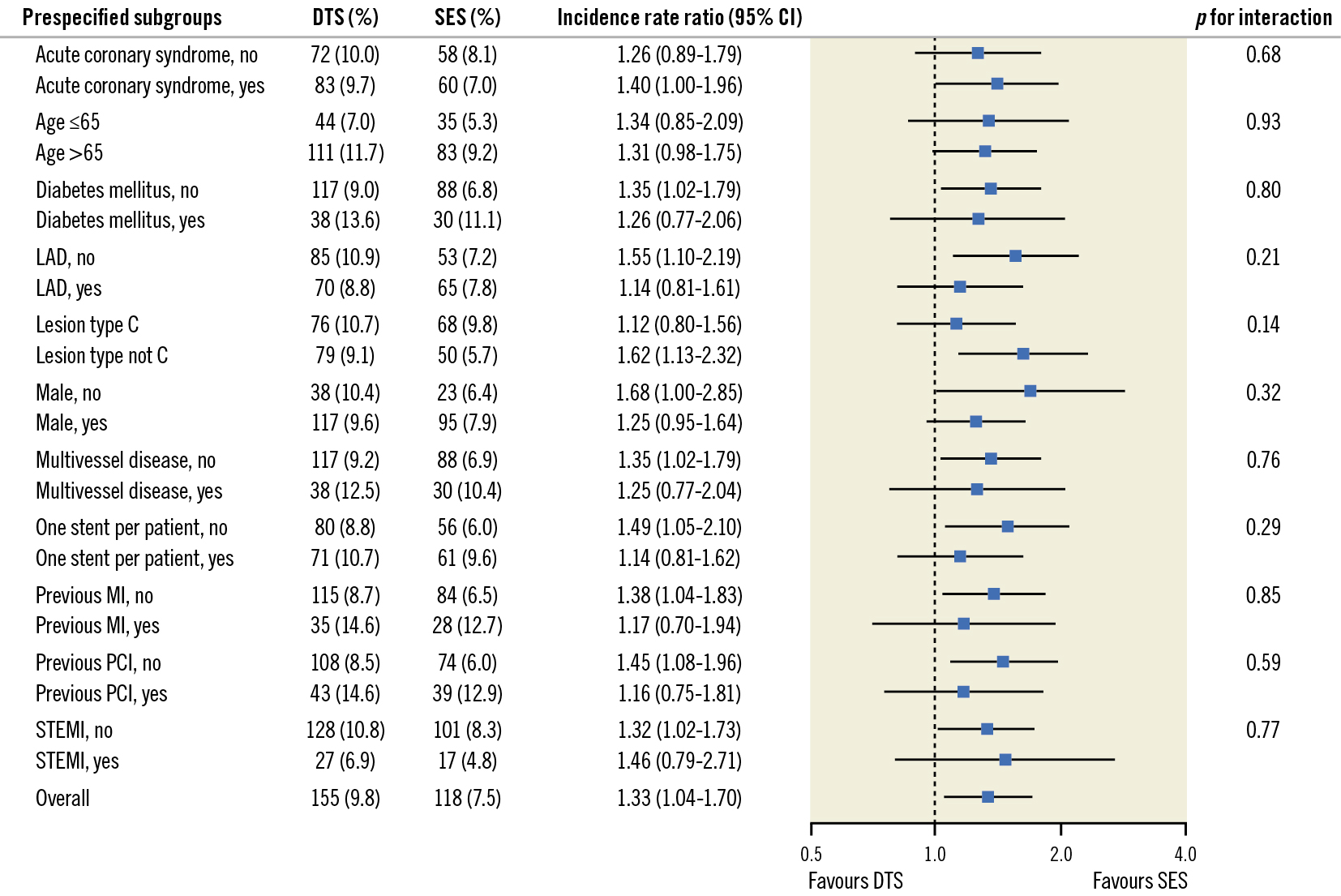
Figure 2. Prespecified subgroup analysis for the primary endpoint at 3-year follow-up. P-values in the forest plot are all 2-sided for interaction. CI: confidence interval; DTS: dual-therapy CD34 antibody-covered sirolimus-eluting stent; LAD; left anterior descending artery; MI: myocardial infarction; PCI: percutaneous coronary intervention; SES: sirolimus-eluting stent; STEMI: ST-elevation myocardial infarction
Discussion
In the SORT OUT X trial, the SES was superior to the DTS at 3 years in an all-comer population. This difference was explained by a significantly higher TLF rate in the DTS group compared to the SES group within the first year, while the TLF rates were almost identical from 1 year to 3 years in both stent groups. The rate of TLR was higher in the DTS group, but the rates of cardiac mortality, MI, and stent thrombosis did not differ significantly between the 2 groups.
In previous randomised controlled trials comparing the DTS with first- and second-generation DES, the DTS was found to be non-inferior234. However, these studies had a maximum of 1-year follow-up and were not powered for clinical outcomes. The only existing long-term data regarding the DTS come from the REMEDEE Registry1516. At 3-year follow-up, TLF occurred in 10.7%, cardiac death in 4.1%, target vessel MI in 2.0% and TLR in 7.1% of patients. These data are in line with our findings. In randomised controlled trials with longer follow-up evaluating the SES, 3-year TLF rates of 8.2-8.9% were reported17181920. Again, these data are in line with our findings.
There are important differences in the DES technologies between the 2 study stents that may have contributed to the early higher TLF rate observed in the DTS group. Most importantly, the DTS has a layer of murine, monoclonal, antihuman CD34 antibody attached to the polymer, which is designed to improve early stent strut coverage. The DTS also has sirolimus attached to the polymer to prevent excess neointima formation. Despite this design, our results suggest that the DTS is associated with early neointimal hyperplasia, causing restenosis in some patients. However, our data also show that the TLF rate and the TLR rate of the DTS from 1-3 years are slightly and non-significantly lower than the TLF and TLR rates of the SES. Interestingly, in the EGO-COMBO Study21, the authors found that the DTS showed a unique late neointimal regression that has not been reported for any other DES; this late neointimal regression was seen between 9 and 24 months. Our results might be explained by this potential benefit of the DTS. The planned 5-year follow-up in the SORT OUT X Study will show whether the TLF rate in the DTS group will be further reduced compared to the SES group in the following years.
Additionally, the study stents have different drug-eluting kinetics. The biodegradable polymer attached to the DTS is completely absorbed within 90 days (compared with 12-24 months for the SES), and the drug release is faster (1 vs 3 months). Based on registry data, Iqbal et al compared the Endeavor stent and the Resolute stent (both Medtronic)22. The two stents were based on the same stent platform; both stents were zotarolimus-eluting stents but had different polymer coatings, resulting in different drug release times. The Endeavor stent released 95% of the drug within 2 weeks, and the Resolute stent released 85% of the drug within 60 days and the remainder by 180 days. The longer drug release time resulted in significantly lower rates of 2-year mortality and TLR. In the present study, the TLR curves started to diverge after 4 months and continued up to 1 year. From 1-3 years, the curves are almost parallel. Early in-stent restenosis is primarily a non-specific inflammatory response to vessel wall injury causing migration of smooth muscle cells from tunica media and myofibroblasts from tunica adventitia to tunica intima. Simultaneously, the vessel discontinuity created by the stent results in neointimal proliferation23. These processes take place within weeks to months. The drugs eluted from the stents aim to counterbalance this excessive neointimal proliferation. Thus, it is possible that these inflammatory processes are still ongoing when the sirolimus from the DTS is completely absorbed, as opposed to a more chronic phase when the sirolimus from the SES is completely absorbed.
Finally, the DTS has thicker stent struts (100 μm) than the SES (60-80 μm). A recent meta-analysis including 10 randomised trials with a total of 11,658 patients compared contemporary second-generation DES versus newer-generation ultrathin-strut DES and concluded that newer-generation ultrathin-strut DES were associated with a 16% reduction in TLF compared to older second-generation thicker-strut DES24.
It is not clear from our results whether our findings are explained by an unintentional effect of the antihuman CD34 antibody attached to the DTS, different drug-eluting kinetics, different stent strut thickness, or other stent-related factors.
Limitations
The SORT OUT X trial, in line with the previous SORT OUT trials121314, relied on registry-based outcome ascertainment without study-related angiographic or clinical follow-up. Patient care complied with standard clinical practice, usually with a single hospital outpatient visit 1 to 3 months after stent implantation. Although the Danish healthcare databases capture events of sufficient severity for patients to seek medical attention, these records might underestimate event rates compared with studies where follow-up is performed by dedicated trial staff. However, any underreporting of events is likely to be low and would not influence the differences detected between treatment groups.
One of the potential benefits of the DTS is early healing and, thus, the potential for shortened dual antiplatelet therapy and reduced bleeding complications. All patients were recommended 6 to 12 months of dual antiplatelet therapy, in accordance with guidelines, but dual antiplatelet therapy adherence cannot be reported. We only reported ischaemic events in the study, and we did not monitor bleeding complications.
Conclusions
After 3 years, the DTS was inferior to the SES, mainly because the DTS was associated with an increased risk of TLR. This difference was caused by a significantly higher TLF rate in the DTS group compared to the SES group within the first year; of note, the TLF rates were almost identical from 1 year to 3 years in both stent groups. The rates of all-cause death, cardiac death, and MI at 3 years did not differ significantly between the 2 groups.
Impact on daily practice
This study provides data on long-term clinical follow-up in patients treated with the DTS compared to the SES. The DTS was inferior to the SES, because the DTS was associated with an increased risk of TLF during the first year but not from 1-3 years. Thus, the sirolimus-eluting and CD34 antibody-covered DTS has no evident advantage when using the guideline-recommended duration of dual antiplatelet therapy.
Funding
The study is supported with equal unrestricted grants from BIOTRONIK, Bülach, Switzerland and OrbusNeich Medical, Fort Lauderdale, FL, USA.
Conflict of interest statement
E.H. Christiansen has received grants from OrbusNeich and Biotronik to his institution. M. Maeng reports personal fees from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Boston Scientific, and Novo Nordisk, outside the submitted work. S.D. Kristensen has received lecture fees from Aspen and AstraZeneca; and research grants from Dorsia. L.O. Jensen has received research grants from Biotronik, OrbusNeich, Biosensors, and Terumo to her institution; and honoraria from Biotronik. The other authors have no conflicts of interest to declare.

