Abstract
Aims: The aim of this study was to examine the five-year clinical outcome in patients enrolled in the Primary Stenting of Totally Occluded Native Coronary Arteries II (PRISON II) study.
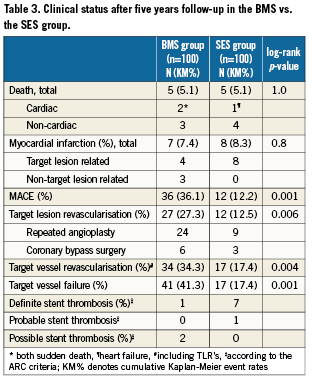
Methods and results: Patients with totally occluded coronary arteries were randomised to either sirolimus-eluting stent (SES, n=100) or bare metal stent (BMS, n=100) implantation. At five years, patients in the SES group had significantly lower rates of target lesion revascularisation (12% vs. 30%, p=0.001), target vessel revascularisation (17% vs. 34%, p=0.009) and major adverse cardiac events (12% vs. 36%, p<0.001). There were no significant differences in death and myocardial infarction. Eight (8%) cases of stent thrombosis (seven definite and one probable; one early, one late, and six very late) were noticed in the SES group versus three cases (3%, one definite and two possible; all very late) in the BMS group (p=0.21).
Conclusions: The results of the present study show that the documented superior short-term angiographic and clinical results of SES in patients with total coronary occlusions are maintained during long-term 5-year follow-up as compared with BMS. On the other hand, there is a trend to a higher stent thrombosis rate in the SES group.
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stent
CCS: Canadian Cardiovascular Society
CTO: chronic total occlusion
DAPT: dual antiplatelet therapy
IVUS: intravascular ultrasound
MACE: major adverse cardiac events
NSTEMI: non-ST-segment elevation myocardial infarction
OCT: optical coherence tomography
SES: sirolimus-eluting stent
ST: stent thrombosis
STEMI: ST-segment elevation myocardial infarction
TCO: total coronary occlusion
TVF: target vessel failure
TVR: target vessel revascularisation
Introduction
Multiple randomised trials have demonstrated that sirolimus-eluting stents (SES) can significantly reduce rates of coronary restenosis and therefore the need for repeated intervention compared with bare metal stents (BMS)1-3. Patients with total coronary occlusions (TCO) have a high risk of restenosis with the use of BMS (between 22% and 55%)4-7. Therefore, we studied whether SES reduced restenosis in this subgroup and randomised 200 patients with TCO to either BMS implantation or SES in the PRISON II trial, and showed a clear superiority of SES over BMS in decreasing angiographic binary restenosis and adverse clinical events, which was maintained up to three years8,9. On the other hand, there was a trend of a higher late stent thrombosis (ST) rate in the SES group at 3-year follow-up. Other reports of very late DES thrombosis, associated with possible increased mortality, have elicited long-term safety concerns10,11.
The present study was undertaken to reveal the very late clinical outcome of the original PRISON II patient cohort and to determine if the safety and efficacy of SES versus BMS in TCO’s were still maintained at five years.
Methods
Patients
The methods of the PRISON II trial have been outlined previously9. In brief, the 200 patients were considered eligible if they had an estimated duration of TCO of at least two weeks with evidence of ischaemia related to the target vessel. Patients were randomised by a telephone allocation service to receive either a conventional BMS (Bx Velocity; Cordis, Johnson & Johnson, Warren, NJ, USA) or aSES (Cypher; Cordis, Johnson & Johnson). All patients previously randomised were asked to participate in this long-term follow-up study. All patients gave written informed consent. The protocol of the study was approved by the institutional ethics committee of the St. Antonius Hospital, Nieuwegein and Onze Lieve Vrouwe Gasthuis Amsterdam, both in The Netherlands. The authors had full access to the data and take full responsibility for its integrity.
All patients were pre-treated with a loading dose of 300mg of clopidogrel and 80mg of aspirin. After the procedure, all patients were prescribed 80-100mg of aspirin indefinitely, and 75mg clopidogrel for at least six months.
Follow-up protocol
All patients included in the PRISON II trial had clinical follow-up at 30 days, 6 months, 1 year, 2, 3, 4, and 5 years. An independent clinical-event committee whose members were unaware of the patient’s treatment assignment reviewed all clinical endpoints during follow-up. Angiography was performed if there were clinical signs of restenosis and, if indicated, was followed by revascularisation. Recurrent angina, a positive exercise test, or abnormal nuclear imaging were considered clinical signs of restenosis. Death, myocardial infarction, and target lesion revascularisation (TLR, defined as ischaemia driven percutaneous or surgical revascularisation of the target lesion due to restenosis within the stent or within 5mm distal or proximal to the stent after the initial procedure) were recorded as major adverse cardiac events (MACE). Target vessel revascularisation (TVR) was defined as repeat revascularisation within the treated vessel, and target vessel failure (TVF) as a composite of death from cardiac causes, myocardial infarction, and ischaemia-driven TVR.
Study endpoints and clinical definitions
This 5-year follow-up study focuses on clinical restenosis, MACE and TVF.
TCO was defined by the absence of antegrade flow of contrast distal to the occlusion (TIMI flow 0 according to the Thrombolysis and Myocardial Infarction [TIMI] score) or only minimal flow of contrast distal to the occluded vessel (TIMI flow 1). The duration of the total coronary artery occlusion had to be at least two weeks and was estimated by clinical information, sequential angiographic information, or both. Chronic total occlusion (CTO) was defined as a coronary occlusion with a duration >3 months according the ACC/AHA lesion classification.
According to the definitions of the Academic Research Consortium (ARC), ST was classified as definite, probable, or possible and as early (0 to 30 days), late (31 to 360 days), or very late (>360 days). The definition of definite ST required the presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion. Probable ST included unexplained deaths within 30 days after the procedure or acute myocardial infarction involving the target-vessel territory without angiographic confirmation. Possible ST included all unexplained deaths occurring at least 30 days after the procedure12,13.
Statistical analysis
The comparison between variables representing counts was assessed with the Fisher’s exact test; normally distributed variables with the Student’s t-test; outcomes with censoring were assessed graphically with Kaplan-Meier curves and statistical hypothesis testing with the log-rank test. Patients were followed for all events until end of study or death. In the tables all events were included without censoring.
All randomised patients were included in the clinical endpoint analyses according to the intention to treat principle. All data were collected, held and analysed by the trial coordination centre at the St. Antonius Hospital, without any involvement of the sponsor. Analyses were performed with the use of R version 2.12.
Results
Baseline clinical and angiographic characteristics
A total of 528 patients were screened for the study. Of these, 62 patients (11.7%) were excluded because the lesion could not be crossed, 14 patients (2.7%) because of spontaneous reperfusion to TIMI flow ≥II, and 252 patients for other reasons. A total of 200 patients were enrolled in the study between January 2003 and September 2004. The median duration of the TCO was 2.8 months.
The two groups were well matched for all baseline and procedural characteristics (Tables 1 and 2).
Long-term clinical outcome
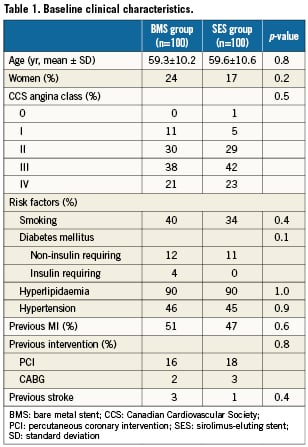
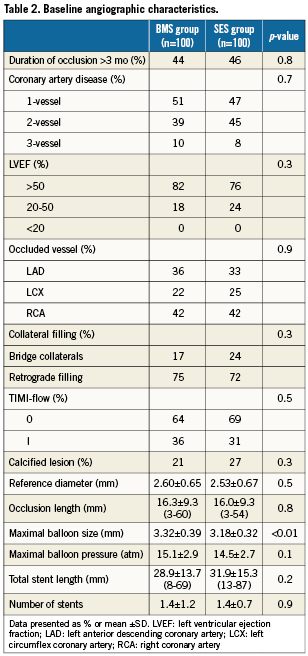
Complete clinical data sets were available at five years in 90% of the patients assigned to the SES group and in 92% of those randomised to the BMS group. Clinical data up to three years of follow-up were published previously8. Between three and five years, one non-cardiac death occurred in each group (one patient died after ischaemic stroke [BMS-group] and one patient died because of a mesothelioma [SES-group]). There were no additional cardiac deaths during this period. For all endpoints, the results of SES were superior to BMS. The cumulative 5-year survival rates free from MACE were 87.8% for the SES-group and 63.9% for the BMS-group (log-rank p=0.001), rates for TLR were 87.5% versus 65.7% (log-rank p=0.006), rates for TVR 82.6% versus 65.7% (log-rank p=0.004) and rates for TVF 82.6% versus 58.7% (log-rank p=0.001) for the SES and the BMS-group, respectively (Figures 1-4 and Table3). Apparently, the benefit of SES in TCO was achieved in the first year as we did not observe significant differences in additional adverse events between one and five years (MACE 10% vs. 10%, TLR 6% vs. 5%, TVR 8% vs. 8% for BMS vs. SES, respectively).
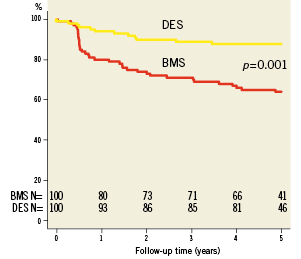
Figure 1. Survival free from major adverse cardiac events (MACE).
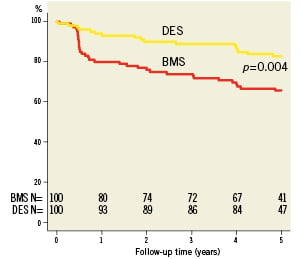
Figure 2. Survival free from target vessel revascularisation (TVR).
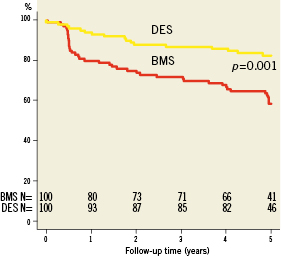
Figure 3. Survival free from target vessel failure (TVF).
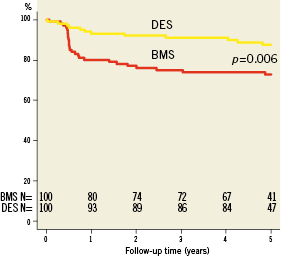
Figure 4. Survival free from target lesion revascularisation (TLR).
Stent thrombosis
A total of eight (8%) stent thromboses was observed in de SES group versus three cases (3%) in the BMS group (p=0.21) after five years of follow-up, including definite, probable, and possible cases. Definite ST occurred in seven patients in the SES group and in one patient in the BMS group (p=0.07). When definite and probable ST were combined the difference became statistically significant despite the small number of patients (1 versus 8 cases, p=0.04). In the SES group, one early definite ST was observed due to local dissection directly distal from the study stent one week after inclusion, leading to a non-ST-segment elevation myocardial infarction (NSTEMI). Another patient without known risk factors for ST developed late ST three months after inclusion despite dual antiplatelet therapy (DAPT). Five patients in the SES group suffered from very late definite ST between 32 and 50 months after the index procedure. One patient, who was on Coumadin was successfully treated with abciximab. Another patient with mild renal failure and a left ventricular ejection fraction of 45% developed ST in a 2.5mm stent which was probably undersized. A third patient presented with aST-segment elevation myocardial infarction (STEMI) and had no known risk factors for the development of ST.
The fourth patient presented with moderate left ventricular function and a long stented segment (41 mm) and was admitted with an inferior wall STEMI. Finally, a patient with a history of diabetes developed ST 52 months after his initial PCI. All patients who suffered very late ST had discontinued clopidogrel therapy and were all on aspirin, except from the patient who was on Coumadin therapy.
In the BMS group, one patient developed ST with STEMI, almost 60 months after the index procedure. It was a 67-year-old male with diabetes mellitus, treated with a 2.5 mm BMS, who had already undergone TLR (balloon angioplasty) because of in-stent restenosis eight months after the index procedure.
Possible ST occurred in two patients in the BMS group because of unexplained sudden death >30 days after stent implantation.
A probable ST occurred between 36 and 48 months in one patient in the SES group who suffered from a NSTEMI, involving the target-vessel territory but without angiographic confirmation. Angiography was performed three days later; stent malapposition was observed with intravascular ultrasound (IVUS) and treated with high-pressure balloon inflation.
Discussion
The current study provides information on the longest available clinical follow-up in the management of patients with a successfully recanalised TCO. The main finding of this study is that the documented superior short-term clinical results of SES in patients with total coronary occlusions are maintained during 5-year follow-up as compared with BMS.
TCO’s remain a major challenge and unresolved dilemma in the practice of interventional cardiology. For example, 11.7% of the patients who were screened for this study were excluded because the lesion could not be crossed. High rates of TVR were noticed after use of balloon angioplasty or BMS14. DES implantation in TCO’s has shown favourable results regarding restenosis rates and clinical outcome in several observational, often retrospective studies15-17. The PRISON II study was the first randomised trial to demonstrate that SES improve both clinical and angiographic outcome in patients with TCO as compared with BMS. Another multicentre, randomised trial was published recently and confirmed the superiority of SES18.
Long-term clinical outcome
Although multiple randomised trials have demonstrated the long-term efficacy and safety of DES compared with BMS in simple denovo lesions19,20, there are limited published data on the long-term outcomes after PCI for “off-label” indications including TCO. Arecent published systematic review and meta-analysis by Colmenarez et al, containing 14 comparative studies (4,394 patients) with a mean clinical follow-up of 22 months, showed that the beneficial effects of DES over BMS were sustained ≥3 years21. They observed significantly fewer rates of MACE (13.5% vs. 28.1%), TVR (11.7% vs. 23.9%), restenosis (10.6% vs. 36.8%), and reocclusion (2.97% vs.10.4%) using DES, without increasing death or myocardial infarction. Han et al demonstrated the superiority of DES (rapamycin- and paclitaxel-eluting stents) to BMS up to five years in a retrospective cohort study22. Our data are consistent with those previous findings. On the other hand, data from the non-randomised RESEARCH Registry showed that the use of SES in CTO treatment was no longer associated with lower rates of TVR and MACE at three and five years follow-up, despite the clinical benefit after one year23,24. This is in line with our observations. Apparently, it seems that the beneficial effect seen with SES on longer follow-up is mainly driven by the reduction in events in the first year, with survival curves running more parallel afterwards.
There have been some concern about the late occurrence of restenosis (“late catch-up phenomenon”) using DES25. Fortunately, reports on long-term follow-up after SES implantation indicate no such rebound phenomenon in simple or complex coronary lesions26. These latter results are consistent with the present study, in which rates of TLR and TVR were similar for SES- and BMS-treated patients during one to five years of follow-up. Thus, it is unlikely that the use of SES is associated with a “late catch-up phenomenon” in treating TCO.
Stent thrombosis
Despite the unequivocal efficacy of DES in reducing the need for repeat lesion revascularisation, there have been serious concerns about long-term safety. Even though, recent systematic reviews and large-scale registries observed similar rates of death and myocardial infarction for patients treated with either a DES or a BMS during long-term four year follow-up27,28. Late and very late ST was encountered steadily at an annual rate of 0.6% for up to four years29. Caixeta et al investigated 5-year clinical outcomes in a pooled analysis of the four SES versus BMS randomised trials, and found a low annual definite or probable ST rate from one to five years which did not differ significantly between SES and BMS (0.4% vs. 0.2% per year). However, very late ST tended to be more frequent in the SES group (1.4% vs. 0.7% ; p=0.02)30.
Compared with on-label use, off-label use of DES is associated with a higher rate of adverse outcomes and late ST31. It has been suggested that the endoluminal surface created after TCO recanalisation constitutes a major challenge for strut endothelialisation with DES use, due to unfavourable features such as the absence of endothelial cells and the exposure of deep plaque components at the site of stenting, the invariable presence of well-developed collaterals, and the frequent need to stent long segments with an increased risk of stent malapposition. Additionally, it has been postulated that there is a higher occurrence of late stent malapposition in SES (and paclitaxel-eluting stents) compared with BMS in patients presenting with very late ST32. In the systematic review by Colmenarez et al, the incidence of ST (definite and probable) was 1.28% in the DES group and 0.39% in the BMS group (p=0.1). Very late ST occurred in seven DES-treated patients (including three PRISON patients) and no BMS-treated patients (p=0.07)21. We observed one late and six very late definite stent thromboses in the SES group and one very late definite ST in the BMS group. There was no association with higher mortality rates and we could not identify obvious additional risk factors for very late ST. Although there were differences in the absolute number of ST and even a significant difference when probable and definite ST were combined , the current study is too small to draw definite conclusions regarding the occurrence of ST in this specific group of patients. Furthermore, the number of ST could be higher than reported because asymptomatic ST may occur in arteries covering a territory with limited myocardial viability. Although the majority of the patients developed ST along time after discontinuation of DAPT, there is no evidence until up now that continued DAPT fully protects against very late ST33. Regarding the higher ST risk in complex lesions, we believe that IVUS and optical coherence tomography (OCT) should be used more frequently to identify stent underexpansion and incomplete stent apposition.
Further studies are required to determine if “next-generation” DES using novel antiproliferative drugs, polymers, and delivery systems, can reduce (very) late ST34.
Limitations
This 5-year follow-up study focuses on MACE, TLR, TVF and ST and is not powered to detect differences in death rate and (very) late ST rates. Secondly, only 45% of the patients had a true CTO with an occlusion duration of more than three months.
Conclusions
In patients with successfully recanalised TCO, clinical outcome five years after SES implantation continues to demonstrate significant reduction in the need for repeat revascularisation with similar safety (death and myocardial infarction). There is no evidence for disproportionate late “catch-up” phenomenon, albeit a trend towards a higher rate of very late stent thrombosis is observed in the DES-treated patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.

