Abstract
Aims: We investigated whether sirolimus-eluting stents (SES) are superior to next-generation zotarolimus-eluting stents (ZES) in treating patients with total coronary occlusions (TCO).
Methods and results: In a prospective, randomised trial we compared the SES with the zotarolimus-eluting stent (ZES; Endeavor or Resolute) after successful recanalisation of TCO. During the first phase of the trial, 51 patients were assigned to receive the SES and 46 patients to receive the Endeavor ZES. In the second phase we randomised 103 patients to the SES group and 104 patients to the Resolute ZES group. The primary endpoint was in-segment late lumen loss at eight-month follow-up. At eight months, patients in the SES group had less in-segment and in-stent late loss as compared to the Endeavor group: –0.13±0.3 mm vs. 0.27±0.6 mm (p=0.0002) and –0.13±0.5 mm vs. 0.54±0.5 mm (p<0.0001), respectively. In contrast, the SES and the Resolute ZES showed comparable amounts of in-segment (–0.03±0.7 mm vs. –0.10±0.7 mm, p=0.6) and in-stent (0.03±0.8 mm vs. 0.05±0.8 mm, p=0.9) late loss.
Conclusions: In the treatment of TCOs, the SES was associated with superior angiographic outcomes compared to the Endeavor ZES. On the other hand, the SES and the Resolute ZES showed comparable angiographic outcomes.
Abbreviations
ARC: Academic Research Consortium
BMS: bare metal stent(s)
CCS: Canadian Cardiovascular Society
CTO: chronic total occlusion
DS: diameter stenosis
IVUS: intravascular ultrasound
MACE: major adverse cardiac events
MI: myocardial infarction
MLD: minimal lumen diameter
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
SES: sirolimus-eluting stent(s)
TCO: total coronary occlusion(s)
TLR: target lesion revascularisation
TVF: target vessel failure
TVR: target vessel revascularisation
VLST: very late stent thrombosis
ZES: zotarolimus-eluting stent(s)
Introduction
The percutaneous coronary intervention (PCI) of total coronary occlusions (TCO) was traditionally limited by high restenosis rates using plain old balloon angioplasty and bare metal stent (BMS) implantation1-3. The introduction of drug-eluting stents (DES) has been associated with a significant decrease in the need for repeat revascularisation in this specific lesion subset4-6. Still, TCOs represent a subgroup of lesions with a higher risk for restenosis7 and an emerging concern regarding very late stent thrombosis (VLST). In the PRISON II study we randomised 200 patients with TCO to either BMS or sirolimus-eluting stent (SES) implantation and demonstrated superior angiographic outcomes at six months and a sustained clinical benefit up to five years in the SES group, despite a higher rate of late and very late stent thrombosis8,9. Whether SES are superior to second-generation zotarolimus-eluting stents in TCO is undetermined. The Endeavor® (Medtronic Inc., Minneapolis, MN, USA) zotarolimus-eluting stent (ZES) consistently reduced the rate of restenosis and the need for repeated revascularisation procedures as compared with a bare metal stent in de novo coronary lesions10,11. On the other hand, the Endeavor ZES seemed to be associated with higher late lumen loss and binary restenosis compared with the SES12,13. This observation could be explained by the nearly opposite vascular healing response of the two stents due to differences in polymer constitution and in kinetics of drug release14,15. The Endeavor ZES promoted rapid and uniform healing of the endothelium and was associated with a reduced occurrence of late acquired incomplete stent apposition, which could contribute to lower rates of late and very late stent thrombosis16,17. The Resolute (Medtronic Inc.) is a next-generation ZES system that employs a novel tri-polymer coating and was designed to match the efficacy and safety of the Endeavor ZES while improving clinical outcomes in more complex lesion subsets18,19. We conducted a prospective, randomised trial to compare the safety and efficacy of SES with ZES (Endeavor and Resolute) in patients undergoing successful recanalisation of a TCO.
Methods
PATIENT SELECTION
The Primary Stenting of Totally Occluded Native coronary arteries III (PRISON III) study is a prospective, randomised, multicentre trial in which 304 patients with TCO were recruited. All patients were treated at one of five high-volume PCI centres. PCI was performed using either the femoral or the radial approach with standard recanalisation and stent implantation techniques. The major goal was to achieve a residual luminal diameter stenosis <30% on visual assessment.
Patients were included if the estimated duration of the total coronary occlusion was at least two weeks with evidence of ischaemia related to the occluded coronary artery (signs of ischaemia found during an abnormal exercise test, defined as ST-segment depression of at least 1.0 mm that is horizontal or downsloping or upsloping ST-segment depression of at least 2.0 mm or signs of ischaemia found during nuclear imaging with exercise, dobutamine, or adenosine, and a reference diameter >2.5 mm). Patients were excluded if the lesion could not be crossed, if the lesion had a complex anatomy making successful stent deployment unlikely, if the guidewire was not in the true lumen distal to the occlusion, in case of sirolimus or zotarolimus allergy, and if the occlusion was situated in a venous or arterial bypass graft. Patients were also excluded if they participated in another trial, if factors were present which made long-term follow-up difficult or unlikely (i.e., a life expectancy <1 year), in case of severe renal failure (creatinine >250 µmol/L), if the patient used Coumadin that could not be stopped before the procedure or if the use of aspirin, clopidogrel and/or heparin was contraindicated.
The study was conducted according to the principles of the Declaration of Helsinki. The medical ethics committees of all sites approved the study protocol, and all patients had to give written informed consent before they underwent the procedure.
RANDOMISATION AND TREATMENT
Randomisation was performed after crossing the lesion, but before initial dilatation. Patients were randomised by a telephone allocation service, which was provided with the randomisation list before recruitment of the first patient. Patients were equally assigned to either the SES or the ZES (Endeavor or Resolute). Halfway through the trial, the Endeavor ZES ceased to be available and was replaced by the Resolute ZES. Thereafter, all study sites used the new Resolute ZES. In case of the necessity of additional stents, only the assigned stent type was used per lesion and/or vessel. Both patient and treating physician were blinded for allocation. Post-dilatation was performed with high inflation pressures in all patients. At the beginning of the procedure patients received a single dose of 10,000 U heparin. All patients received aspirin and clopidogrel before the procedure, with clopidogrel (75 mg/day) continuing for 12 months and aspirin (80-100 mg/day) lifelong.
Stent systems
The SES (CYPHER™; Cordis Corporation, Miami Lakes, FL, USA) comprises a stainless steel stent which is pre-mounted on a Duralyn© (Cordis) delivery balloon. The stent is covered with sirolimus and a composite PEVA/PMBA permanent polymer. Sirolimus is a macrolide antibiotic that binds to the cytosolic receptor FKBP12 and inhibits down-regulation of the cyclin-dependent kinase inhibitor, thereby inhibiting vascular smooth muscle proliferation and migration. The sirolimus content on the stent is 140 µg/cm2. The maximal drug load on the stent is 419 µg for the largest stent (4.0×33 mm). Most of the drug is eluted from the polymer coating by 28 days and fully eluted by 60 days20.
The Endeavor ZES (Medtronic, Minneapolis, MN, USA) is based on a cobalt alloy stent, pre-mounted on the RX-delivery system and is covered with a permanent biocompatible phosphorylcholine polymer. Zotarolimus is a close structural analogue of rapamycin, which differs only in the presence of a tetrazole group at position 42. It is an immunosuppressant that has extremely low water solubility. The zotarolimus load of the stent is 10 µg/mm stent length. The maximal drug load on the stent is 300 µg for the largest stent (4.0×30 mm). Approximately 95% of the drug is eluted from the stent within 15 days, although drug concentrations may be detected as late as 30 days after stent deployment21.
The Resolute ZES (Medtronic) is the next-generation ZES, which is similar to the Endeavor ZES, except that the biomimetic phosphorylcholine polymer is replaced with the BioLynx polymer system. The BioLynx system consists of a blend of three different polymers: 1) the hydrophobic C10 polymer, which aids in the control of drug release; 2) the hydrophilic C19 polymer, which supports biocompatibility; and 3) polyvinyl pyrrolidinone, which increases the initial drug burst and enhances the elution rate. The BioLynx coating enables finer control of drug elution: 85% of its zotarolimus content is eluted during the first 60 days; the remainder is completely eluted by 180 days. The hydrophilic surface mimics the body’s biological chemistry, thereby reducing the risk of an inflammatory response22.
OBSERVATIONS AND FOLLOW-UP
Coronary angiograms were performed at baseline, immediately after the procedure, and at the eight-month follow-up, using the same views at all times. All angiographic images were digitally recorded and assessed off-line at an independent angiographic core laboratory (St. Antonius Hospital Angiographic Core Laboratory, Nieuwegein, The Netherlands) with an automatic edge-detection system (CMS version 5.3; Medis Medical Imaging Systems, Leiden, The Netherlands) by experienced personnel who were not provided with any clinical information or the type of stent implanted. Before angiography, 100-300 µg nitroglycerine was given intracoronary. The non-tapered tip of the catheter was used as the calibration standard. All lesions were assessed in at least two orthogonal views and the projection showing the smallest diameter (worst view) was used for quantitative coronary angiography (QCA) analysis. Views with the least foreshortening were used for measuring the length of the occlusion. In disease-free proximal segments the reference diameter was measured. Recurrent angina, a positive exercise test or abnormal nuclear imaging were considered as clinical signs of restenosis. Follow-up angiography was performed earlier if there were clinical signs of restenosis and, if indicated, followed by target lesion revascularisation (TLR). Any coronary angiography performed within four months after the initial procedure was considered unscheduled. When an unscheduled angiography was followed by TLR or target vessel revascularisation (TVR), no further angiogram was needed. If no revascularisation took place, repeat angiography at eight months was still required. If the angiography took place after four months, eight-month angiographic assessment was not mandatory.
Quantitative measurement included the reference diameter of the vessel, the minimal lumen diameter (MLD), percentage diameter stenosis (DS) and late lumen loss. QCA was used to evaluate the stented area (in-stent) and the area that included the stented segment as well as the 5 mm margins proximal and distal to the stent (in-segment). Angiographic binary in-stent restenosis was defined as at least 50% residual DS within the stent. In-segment binary restenosis was defined as at least 50% residual DS located in the stent and/or at the 5 mm proximal or 5 mm distal edge. Re-occlusion was defined as a recurrent total occlusion at the previous angioplasty site.
Clinical follow-up was performed at one, six and 12 months, with annual evaluation up to five years. An independent clinical events committee, members of which were unaware of the patient’s treatment assignment, reviewed all clinical endpoints during follow-up. Death, myocardial infarction (MI), or TLR were recorded as major adverse cardiac events (MACE). In addition, occurrences of angina (Canadian Cardiovascular Society, CCS class), TVR and target vessel failure (TVF) were recorded. The incidence of stent thrombosis was recorded according to the definitions of the Academic Research Consortium (ARC).
ENDPOINTS AND DEFINITIONS
The primary endpoint was in-segment late lumen loss at eight months as assessed by an independent angiographic core lab. Secondary endpoints included the following: in-stent late lumen loss, binary in-stent and in-segment restenosis rate, in-stent and in-segment MLD and percentage DS at eight-month follow-up, and MACE, a composite of death, MI and clinically driven TLR (defined as percutaneous or surgical revascularisation of the target lesion after the initial procedure), the individual components of the composite endpoint, stent thrombosis (acute, <1 day; subacute, one to 30 days; and late, >30 days), TVR, defined as repeat revascularisation within the treated vessel, and TVF, defined as a composite of death from cardiac causes, MI, and ischaemia-driven TVR, up to five years.
MI was defined as the presence of new significant Q-waves or an elevation of creatine kinase or its MB isoenzyme to at least two times the upper limit.
According to the ARC definitions, stent thrombosis was defined as “definite” in case of an acute coronary syndrome with angiographic documentation of either occlusion of the target lesion or thrombus within or adjacent to a previously stented segment. Stent thrombosis was defined as “probable” if a sudden unexplained death occurred within 30 days or if a target-vessel-related MI occurred without angiographic documentation. Stent thrombosis was defined as “possible” if a sudden unexplained death occurred after 30 days that could not be attributed to another cause23.
TCO was defined by the absence of an antegrade flow of contrast distal to the occlusion (Thrombolysis in Myocardial Infarction [TIMI] flow 0 according to the TIMI score), or only minimal flow of contrast distal to the occluded vessel (TIMI flow I). The duration of the TCO had to be at least two weeks and was estimated by clinical information, sequential angiographic information or both. Chronic total occlusion (CTO) was defined as an occlusion with a duration of >3 months according the ACC/AHA lesion classification. A post hoc subgroup analysis was performed to examine the difference between patients with CTO and non-chronic total occlusion (nCTO), defined as TCO with an estimated duration of less than three months. The estimated length of the occlusion was measured from the proximal point of the total occlusion to the most distal point of the lesion, which was visualised with the first contrast injection after successful recanalisation. The total coronary analysis segment was defined as the stented segment including the margins 5 mm distal and proximal to the stent.
STATISTICAL ANALYSIS
The original objective of the study was to assess whether the outcome of treatment with the SES is superior to the outcome of treatment with the Endeavor ZES. Initial calculation of the sample size was based on a clinically relevant difference of in-segment late lumen loss of 0.25 mm. This value is equal to 35% of the standard deviation of the late loss in the SES group in the PRISON II study (0.72 mm), which is a margin of clinically relevant measure used in, for instance, the ISAR-DIABETES study24,25. Using a two-sided alpha level of 0.05, we estimated that 132 patients per group were needed to demonstrate superiority of the SES relative to the Endeavor ZES with a statistical power of more than 80 percent in a Student’s t-test. To allow for loss of follow-up coronary angiography, 150 patients in each group were planned as necessary.
After the Endeavor ZES was replaced by the Resolute ZES, the steering committee decided to split the trial into two phases: the SES versus Endeavor ZES comparison (phase 1) and the SES versus Resolute ZES comparison (phase 2). At a pre-planned interim analysis, the data and safety monitoring committee (DSMB) reached the conclusion that the study objective for phase I (superiority of the SES relative to the original Endeavor ZES) had been met. The available study data for phase 2 indicated that the original objective (superiority of SES versus ZES) was unlikely to be met in this phase. The steering committee then decided to complete the trial according to protocol and to consider phase 2 as an exploratory study. If the second phase is interpreted as a non-inferiority trial, phase 2 (2×80 patients) would have a statistical power of 80% to establish non-inferiority with a margin of 0.30 mm and a standard deviation of 0.74 mm. Fisher’s exact test for categorical variables was one of the statistical tests employed and all statistical evaluations were performed in R (version 2.15 for Windows®; http://www.r-project.org).
Results
PATIENTS
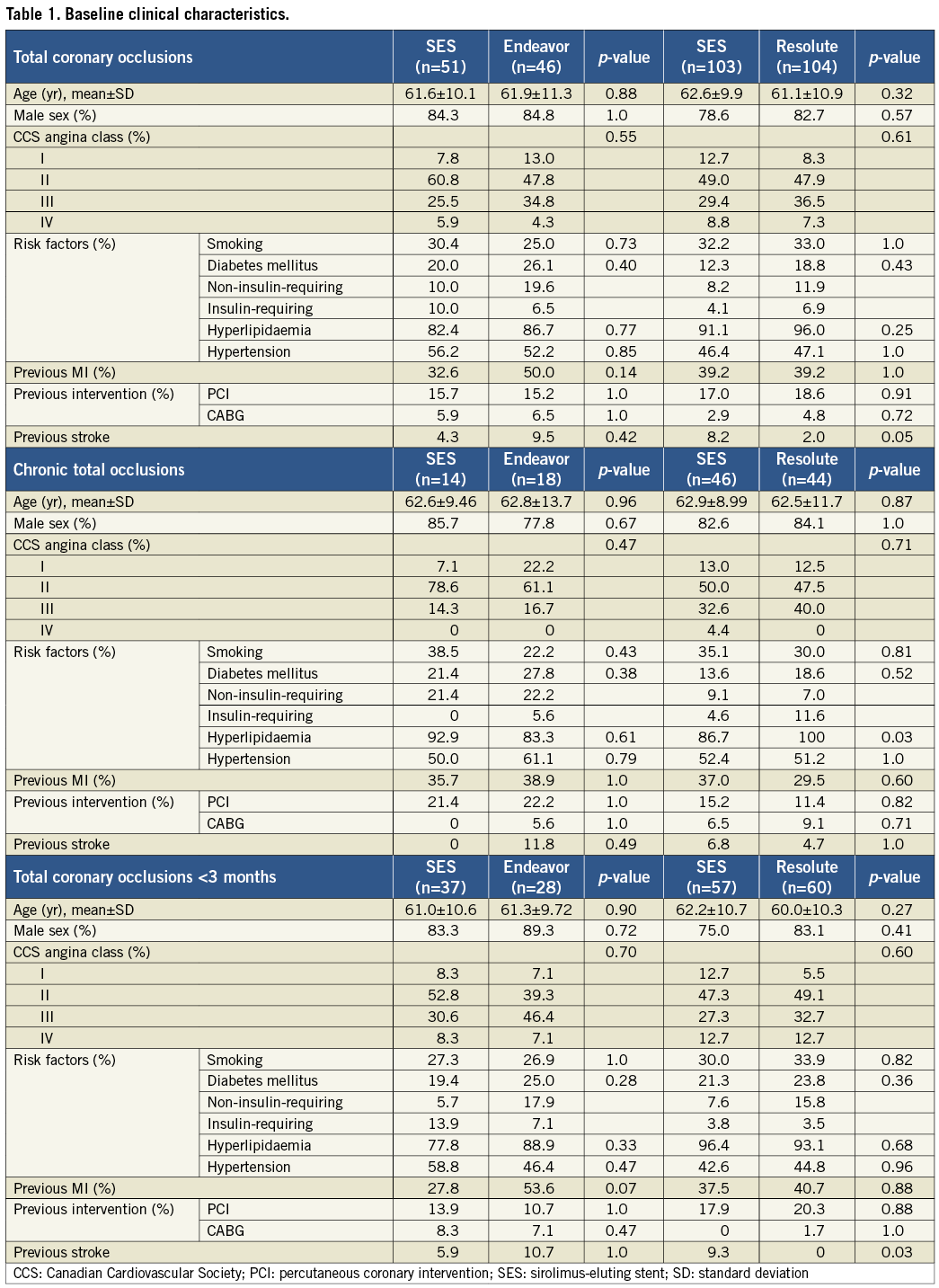
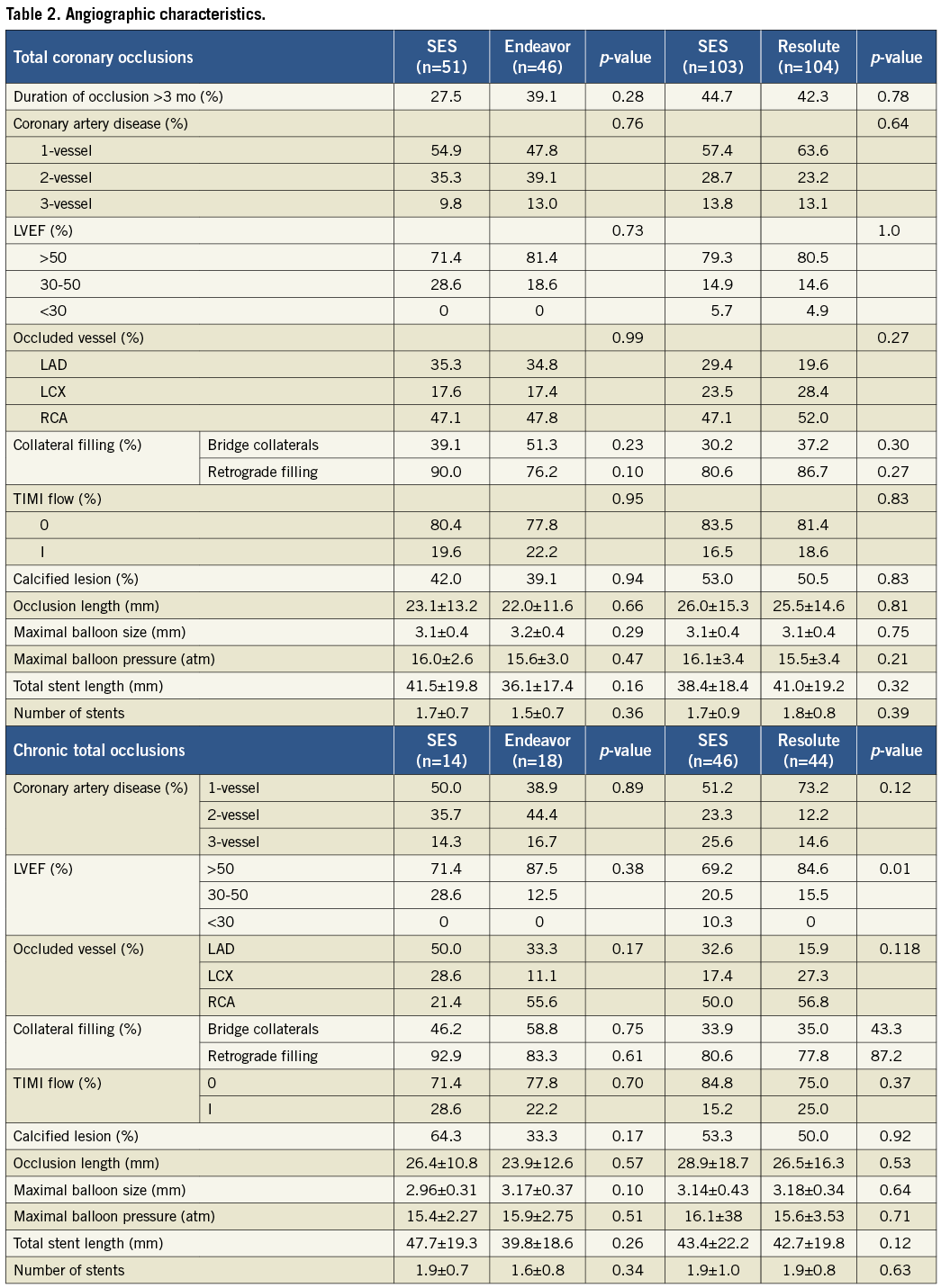
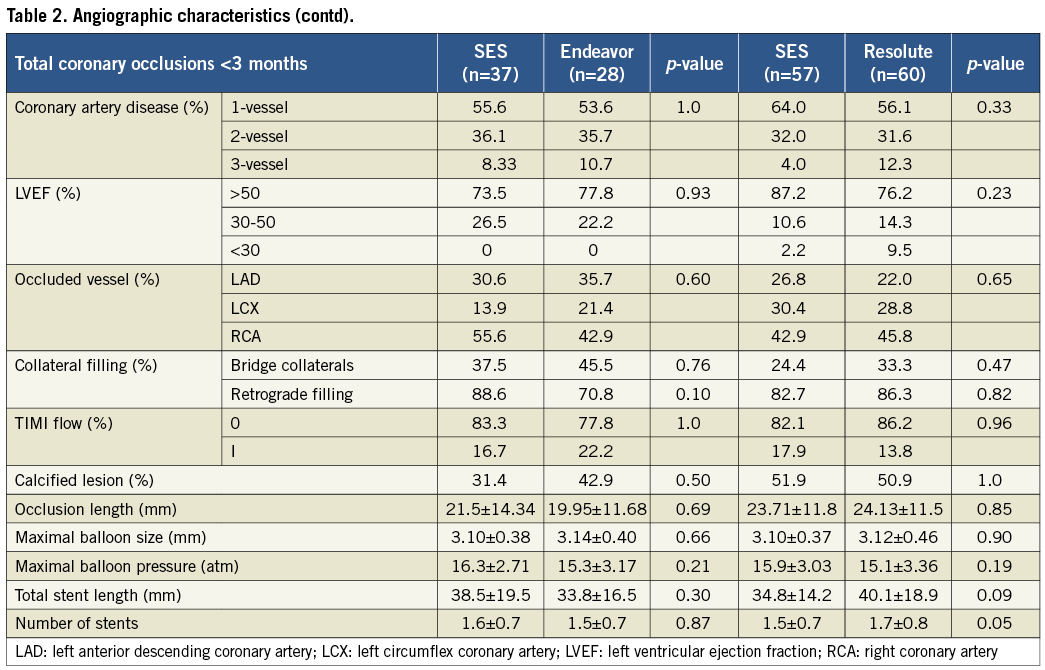
A total of 383 patients were eligible for enrolment in the study from January 2007 to December 2010. Of these, 304 patients were successfully recanalised and randomised in two study phases (Figure 1): 97 patients were assigned to the SES versus the Endeavor ZES comparison (phase 1) and 207 patients to the SES versus the Resolute ZES comparison (phase 2). No significant differences were present in the baseline clinical and angiographic characteristics between the groups, except that fewer patients had a previous stroke in the Resolute ZES group compared to the SES group in phase 2 (Table 1 and Table 2). In total, angiographic follow-up at eight months was obtained in 239 patients (75% in the SES group of phase 1, 78% in the Endeavor ZES group, 78% in the SES group of phase 2, and 82% in the Resolute ZES group). All the missing patients refused follow-up angiography and were asymptomatic. No adverse events were observed among these patients. All QCA parameters are displayed in Table 3. All patients completed the 12-month clinical follow-up. The clinical events during both phases of the trial are shown in Table 4.
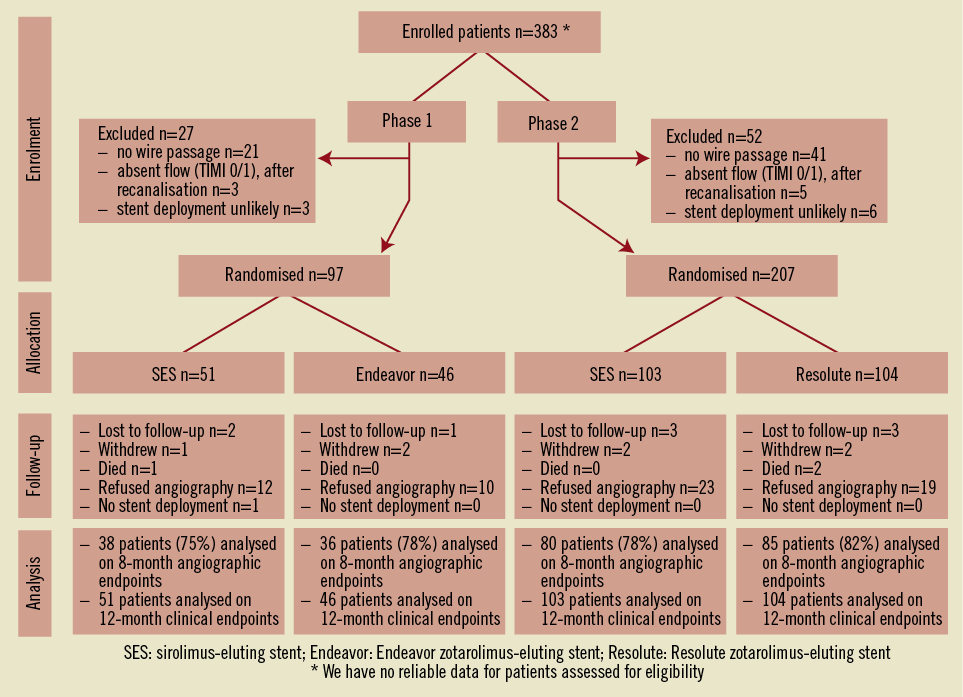
Figure 1. Study enrolment and randomisation.
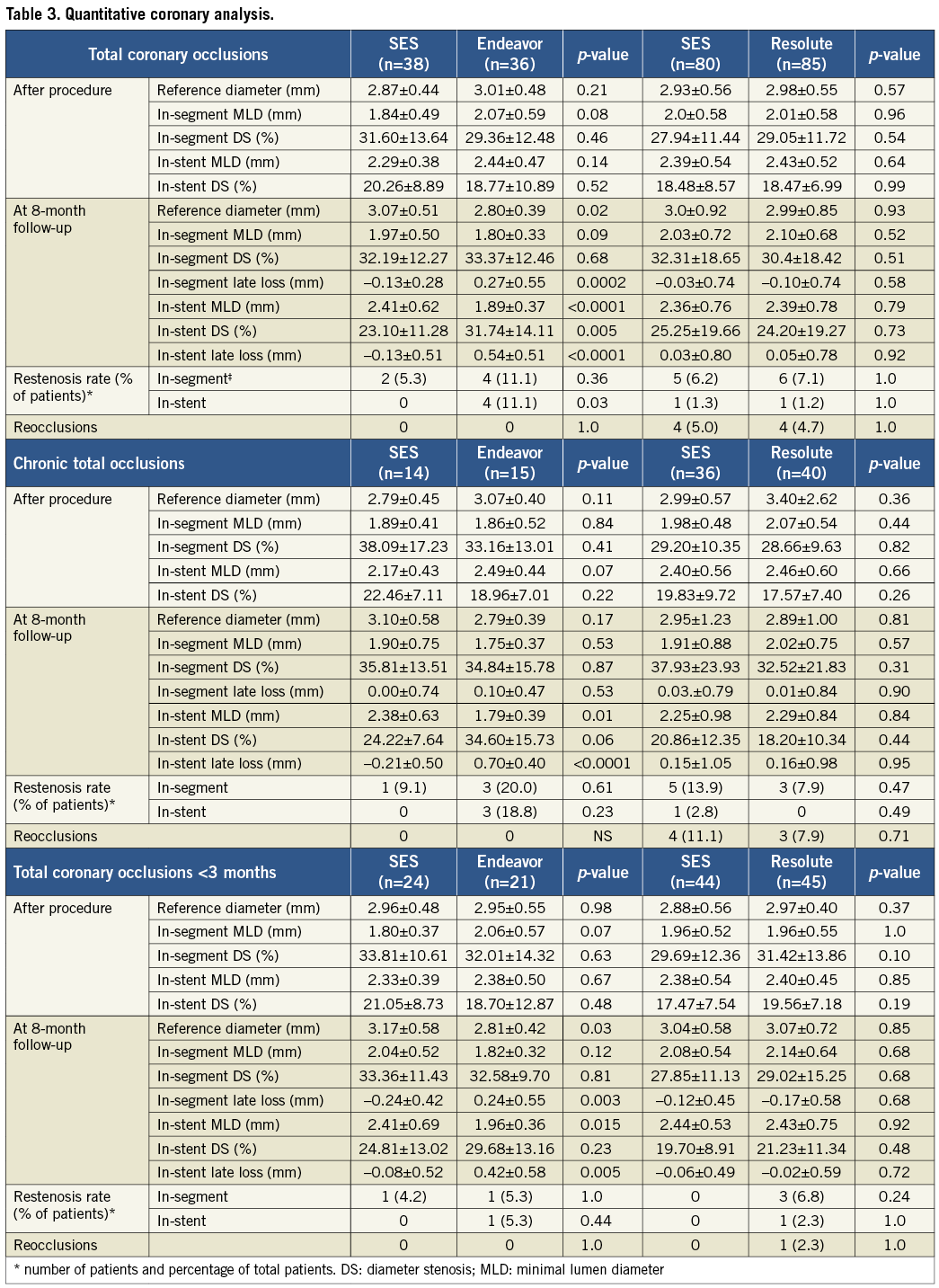
SES VERSUS ENDEAVOR ZES COMPARISON
The primary endpoint, in-segment late lumen loss, was significantly higher among patients treated with the Endeavor ZES versus the SES (0.27±0.6 mm vs. –0.13±0.3 mm, p=0.0002). Both in-stent late lumen loss and in-stent binary restenosis were also significantly higher in the Endeavor ZES group: 0.54±0.51 mm vs. –0.13±0.51 mm (p<0.0001) and 11.1 vs. 0% (p=0.03), respectively.
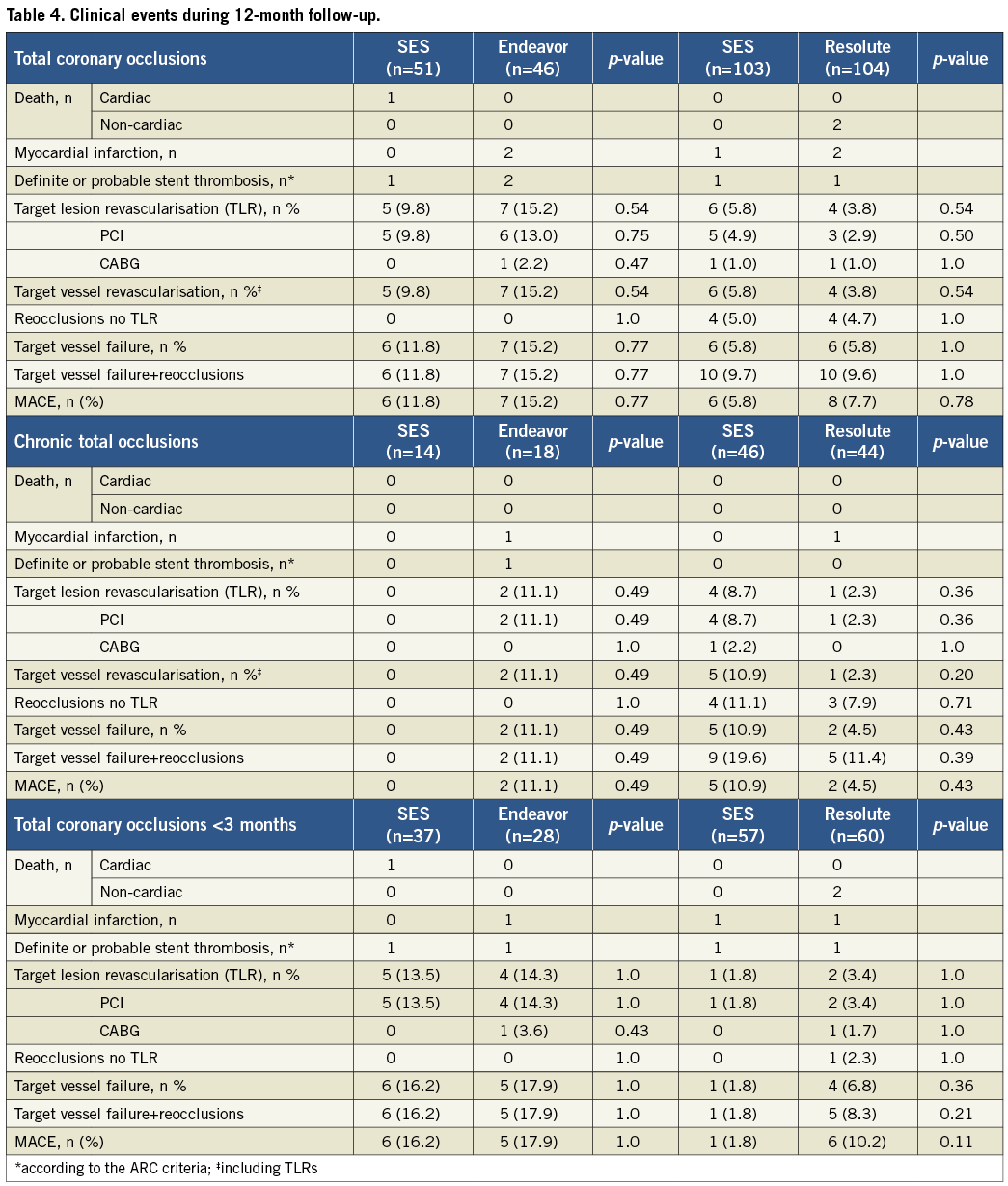
One patient (2%) in the SES group died suddenly one day after the index procedure. The aetiology could not be discovered and was therefore classified as probable stent thrombosis (ST; 1.9%). No patients died in the Endeavor ZES group. A target-lesion-related MI was present in two patients (4.3%) in the Endeavor ZES group. Both MIs were classified as one probable and one definite ST (4.3%). At 12 months there were no significant differences between SES and Endeavor ZES in the occurrence of MACE (11.8% vs. 15.2%, p=0.8), TLR (9.8% vs. 15.2%, p=0.5), and TVF (11.8% vs. 15.2%, p=0.8).
SES VERSUS RESOLUTE ZES COMPARISON
The primary endpoint, in-segment late lumen loss, was comparable between SES and Resolute ZES (–0.03±0.7 mm vs. –0.10±0.7 mm, p=0.6). The treatment difference was 0.06 mm with 95% confidence interval from –0.16 to 0.29 (p=0.58). This result meets the post hoc defined criterion for non-inferiority. In-stent late lumen loss was similar between SES and Resolute ZES (0.03±0.8 mm vs. 0.05±0.8 mm, p=0.9). Furthermore, the percentages of in-segment and in-stent binary restenosis were comparable: 6.2% vs. 7.1% (p=1.0) and 1.3% vs. 1.2% (p=1.0), respectively.
There were no cardiac deaths in either treatment group. In the Resolute ZES group, one patient died from duodenal carcinoma and another from metastatic lung cancer, respectively nine and 12 months after the procedure. One patient in both groups suffered a target-related MI and one patient in the Resolute groups suffered a non-target-related MI.
During the 12-month follow-up, we recorded no differences between the study groups regarding MACE (5.8% vs. 7.7%, p=0.8), TLR (5.8% vs. 3.8%, p=0.5), TVR (5.8% vs. 5.8%, p=1.0), or stent thrombosis (1.0% in both groups).
CHRONIC VERSUS NON-CHRONIC TOTAL OCCLUSIONS
The lower panels of Table1-Table 4 show the results of the subgroup analysis by chronicity of the total occlusion. The prevalence of CTO was 28.0% and 39.1% with SES versus Endeavor ZES, and 45.1% and 42.7% with SES versus Resolute ZES, respectively. Patients with CTO were older and presented with more insulin-requiring diabetes mellitus, multivessel disease, bridging collaterals and longer lesions. As a result, more stents were used with longer stent lengths compared to patients with nCTO. Repeated angiography in CTOs demonstrated a higher degree of late lumen loss with an increased rate of binary restenosis and reocclusions compared to nCTOs. Angiographic results remained more favourable with SES compared to Endeavor ZES in both CTOs and nCTOs, except for in-segment late lumen loss in CTOs. In the SES and Resolute ZES comparison, late lumen loss was comparable in both CTOs and nCTOs. The occurrence of clinical events at 12 months was higher in the CTO group with SES versus Resolute ZES. On the contrary, in the SES versus Endeavor ZES comparison, the occurrence of clinical events was lower in the CTO group.
Discussion
The objective of the Prison III trial was to assess whether treatment with the first-generation SES was superior to treatment with the second-generation ZES after successful recanalisation of a TCO. Although the study became underpowered to demonstrate superiority of the SES relative to the Endeavor ZES and the Resolute ZES, we clearly showed a significantly higher late lumen loss in the Endeavor ZES group compared to the SES group. We therefore concluded that the first phase of the trial had met its endpoint, in spite of the relatively small study size. In the second phase we showed that the use of the SES and the Resolute ZES in TCO was associated with comparable angiographic outcomes.
The study was not adequately powered to examine differences between clinical endpoints.
Patients with TCO undergoing PCI have a high risk of angiographic restenosis and the subsequent need for repeat revascularisation26. Both first-generation DES, the SES and the paclitaxel-eluting stent (PES) have been shown to be safe and effective in reducing the incidence of restenosis and TLR in those patients27. Only SES have been tested in a randomised setting and showed a clear superiority in comparison with BMS28,29. Until now, no second-generation zotarolimus-eluting stent has been evaluated in patients with successfully recanalised TCO. The Endeavor ZES showed favourable angiographic and clinical results in de novo coronary lesions and in real-world practice, even beyond 12-month follow-up30-33. The Endeavor III trial randomised (3:1) 436 patients with native coronary artery disease to receive SES or ZES. At eight months, treatment with ZES seemed to be associated with significantly higher late lumen loss compared to SES (in-segment 0.34±0.44 mm vs. 0.13±0.32 mm and in-stent 0.6±0.48 mm vs. 0.15±0.34 mm, p<0.001), although differences in clinical outcome were less consistent34. In contrast, in the SORT OUT III trial, which compared SES and ZES in routine clinical practice (including 34% type C lesions), ZES was associated with higher rates of MACE (10% vs. 5%, p<0.0001) after 18-month follow-up. In TCO, we observed a similar excess of late lumen loss in the ZES group at eight-month follow-up with a slight statistically non-significant increase in adverse clinical events compared to the SES-treated patients35. One of the reasons for the increased neointimal hyperplasia observed with ZES is probably due to the more rapid elution kinetics of zotarolimus and therefore shorter tissue exposure to the drug21. On the other hand, IVUS and OCT studies have shown that a relatively larger amount of more evenly distributed neointima in ZES is associated with more effective strut endothelialisation, compared to SES36,37. Especially in TCO, with the absolute absence of endothelial cells and the frequent need to use stent long segments, this may offer a protective advantage against stent thrombosis. Given these observations, the goal of DES treatment should be to balance the benefits of restenosis prevention against the risks of delayed healing and late stent thrombosis, which will be most challenging in complex lesions like TCO.
The next-generation ZES, the Resolute, uses a new proprietary coating that extends the duration of zotarolimus delivery to match the prolonged healing duration often experienced in more complex cases38. In single, de novo coronary lesions, the Resolute stent demonstrated low late lumen loss at nine months (in-segment 0.12±0.27 mm, in-stent 0.22±0.27 mm), low rates of MACE and TLR at the two-year follow-up, and no late stent thrombosis39. The recently published two-year results from the Resolute All Comers trial confirmed the good safety profile of the stent in an unrestricted patient population40. Furthermore, an IVUS study demonstrated a much more potent suppression of neointimal hyperplasia compared to the Endeavor ZES and a similar neointima growth compared to the SES41. We are the first to compare the safety and efficacy of the Resolute ZES to the SES in TCO. Overall, the Resolute stent demonstrated comparable angiographic and clinical outcomes, after eight and 12 months, respectively. Both stent systems were associated with very low amounts of in-segment and in-stent late loss. These data support the hypothesis that the extended release of zotarolimus from the BioLinx polymer results in low late loss, despite the presence of “restenosis-triggers” in our study population. Although we only observed a few probable and one late definite stent thrombosis, the potential drawback of this gradual elution is incomplete strut endothelialisation and the occurrence of very late stent thrombosis (VLST).
In the PRISON II trial, we demonstrated 6% VLST after five years in the patient group treated with SES9. The pathological mechanism of VLST remained unresolved. However, incomplete vessel healing due to late acquired incomplete stent strut apposition, chronic inflammation and positive vessel remodelling have been proposed as a possible cause33. Incomplete vessel healing after SES was also suggested by detecting peri-stent contrast staining (PSS) at repeated angiography in the j-Cypher registry34. Peri-stent contrast staining was related to stent fracture and mostly observed with SES in CTOs, long lesions and lesions in coronaries with large reference diameters. More importantly, the occurrence of PSS was associated wth TLR and VLST. In this study, we did not investigate the occurrence of PSS or stent fracture and we have to await our long-term follow-up results to observe the possible occurrence of late catch-up and VLST.
Finally, this study involved a combined population of patients with CTOs and nCTOs. Approximately 40% of the patients had a true CTO with an estimated occlusion length of 28 mm and a total stent length of 43 mm. These lesion characteristics are comparable to true CTOs reported in other CTO registries35-37. In CTOs, we observed a higher degree of late lumen loss. However, the efficacy of all investigated stents was similar in both CTOs and nCTOs.
In contrast to the results during the BMS era42, the use of the three different DES systems in our study seems to provide similar angiographic and clinical results in TCO and in de novo lesions. According to our findings, we must state that both the SES and the Resolute ZES are safe in treatment of TCO and are associated with low rates of adverse events due to their potent inhibition of restenosis. Future randomised study designs have to determine if other next-generation DES might be more effective for this specific indication43.
Study limitations
There are several limitations that need to be acknowledged. The split of the study in two was not pre-planned but driven by the availability of the Endeavor and Resolute stents. As a result both studies are relatively small. Due to this fact, the results of the trial should be considered as hypothesis-generating. The lack of power in the first phase was compensated by a larger treatment effect. The post hoc non-inferiority design of the second phase necessarily utilised a wide non-inferiority boundary.
Furthermore, taking an angiographic primary endpoint, the conclusions of our trial are based on incomplete observations: 78% angiographic follow-up in the current study. Nevertheless, the percentages of angiographic follow-up were comparable between the stent groups.
Finally, only 40% of patients had a true CTO. Therefore, the results of the total study population cannot be extrapolated to only patients with CTO. Our subgroup analysis clearly shows the results for patients with a true CTO.
Conclusions
Implantation of an SES or a Resolute ZES after successful TCO recanalisation resulted in a favourable and comparable antirestenotic efficacy and safety profile. Endeavor ZES implantation was associated with a greater amount of neointimal hyperplasia compared to SES. Both SES and Resolute ZES can be recommended in the treatment of TCO.
Funding
This study was supported by an unrestricted research grant from the Cordis Corporation.
Conflict of interest statement
J. J. Koolen received research grants from Biotronik SE & Co. KG (Berlin, Germany) and J.P. Henriques received research grants from Abbott Vascular (Santa Clara, CA, USA) and Abiomed (Danvers, MA, USA). All other authors have no conflicts of interest to declare.

