Abstract
Chronic total coronary occlusions constitute a sub-group of lesions at very high risk of restenosis after successful percutaneous coronary intervention. The sirolimus-eluting coronary stent is the only drug-eluting stent that has demonstrated to reduce angiographic restenosis and the need for new revascularisation procedures in comparison with bare-metal stents in randomised clinical trials focusing on these lesions. Everolimus-eluting stents have shown to offer optimal angiographic and clinical outcomes in comparison with bare-metal stents and paclitaxel-eluting stents, but no randomised trials have tested the device in chronic total occlusions. The CIBELES (non-acute Coronary occlusIon treated By EveroLimus-Eluting Stent) will randomise 208 patients with chronic total coronary occlusions in 13 centres from Portugal and Spain to receive everolimus- or sirolimus-eluting coronary stents. The primary endpoint will be angiographic in-stent late loss.
Rationale of the CIBELES trial
DES have revolutionised interventional cardiology, because they dramatically reduced the risk of both binary angiographic restenosis, and new revascularisation procedures in comparison with bare-metal stents (BMS)1. Currently, there are more than 20 different types of DES commercially available worldwide. However, until recently only the sirolimus-eluting (SES) Cypher coronary stent (Cordis, Johnson & Johnson, Warren, NJ, USA), and the paclitaxel-eluting (PES) Taxus coronary stent (Boston Scientific, Natick, MA, USA) have demonstrated clear clinical benefit at long-term (4-5 years) in large randomised trials and in a wide variety of clinical, and angiographic scenarios1,2.
The risk of angiographic restenosis and the need for new revascularisation procedures after percutaneous coronary interventions (PCI) is especially high in patients with chronic total occlusions (CTO)3. Although coronary stents reduce the risk of restenosis in comparison with balloon alone angioplasty in this subset of patients, the rates of restenosis and vessel re-occlusion is still very high after BMS implantation (~40%, and ~7%, respectively)4. In CTO, only SES have been tested in randomised trials in comparison with BMS. In the PRISON-II trial, treatment with SES, significantly reduced the rates of binary angiographic restenosis (from 41% to 11%, p<0.01), vessel re-occlusion (from 13% to 4%, p=0.04), and the need for new revascularisation procedures (from 22% to 8%, p<0.01) in comparison with BMS5. Apart from SES, no other DES type has been evaluated in randomised trials focused on treatment of CTO. Some non-randomised registries have shown good outcomes after PES implantation in patients with successfully recanalised CTO6, but with apparently poorer results than those obtained with SES7.
Everolimus is an analogue of rapamycin with anti-proliferative and immunosuppressant properties. Coronary stents eluting everolimus (EES) have shown to be effective in reducing angiographic restenosis and the need for new revascularisation procedures in comparison with BMS in three small randomised trials including angiographically and clinically favourable patients (FUTURE-I, FUTURE-II, and SPIRIT-I)8,9. Other randomised trials have compared EES, and PES. In the SPIRIT-II trial, 300 patients were randomly allocated to EES (n=223) or PES (n=77). In-stent late loss (ISLL) at six months was significantly lower with EES, and the incidence of major adverse cardiac events (MACE) at one year was 2.7%, and 9.2% with EES and PES, respectively (p=0.04)10. The SPIRIT-III trial compared EES and PES in 1,200 patients with native coronary lesions. ISLL was lower in patients allocated to EES, and the incidence of MACE at eight months was 4.6% vs. 8.1% with EES, and PES, respectively (p=0.03)11. More recently, the clinical benefits of EES in comparison with paclitaxel-eluting stents have been demonstrated in the SPIRIT-IV and COMPARE trials, in which a significant reduction in the rate of target lesion failure and major adverse cardiac events was found in patients allocated to EES12,13. EES has not been compared with SES in randomised trials, although indirect data suggest that their efficacy might be comparable in angiographic and clinical terms2,12.
Both SES and EES provide very low ISLL values and low rates of binary angiographic restenosis, and new revascularisation procedures2 and thus not only SES but also EES probably may offer very good angiographic and clinical results in CTO. In addition, EES is made from a cobalt-chromium alloy that provides a more flexible platform in comparison with stainless steel coronary stents15. However, EES has so far only been tested in relatively favourable lesions and patients, whereas trials in an unfavourable scenario, such as CTO, are lacking. Some non-randomised comparisons have shown apparently similar efficacy of SES and EES (and possibly superiority in comparison with PES and zotarolimus-eluting stents) in the treatment of CTO16.
The CIBELES (non-acute Coronary occlusion treated By EveroLimus-Eluting Stent) trial (Clinicaltrials.gov identifier: NCT00793221) will compare the EES (Abbott Vascular, Redwood City, CA, USA ), versus SES in patients with CTO. Everolimus (40-O-[2-hydroxietil]-rapamycin) (Novartis Pharmaceuticals Corp., Basil, Switzerland), is a new semi-synthetic macrolide with immunosup-pressant properties. The platform of the ESS stent is the cobalt-chromium alloy Multi-Link Vision or Mini-Vision (CE no. 71619). This stent platform is covered by two polymers approved for other applications where there is contact with blood. The promoter of the trial is the Spanish Society of Cardiology, supported by an unrestricted grant from Abbott Vascular, and Chiltern International will be the Clinical Research Organisation (CRO) managing the study conduct and employed by the SEC.
The primary endpoint of the CIBELES trial will be in-stent late loss (ISLL) at angiographic follow-up (nine months). In the era of DES, the rate of binary endpoints (e.g., binary angiographic restenosis and target vessel revascularisation) has been reduced dramatically, and thus the number of patients required to be included in randomised trials would be extremely high. ISLL is a continuous variable that allows a reduction in the number of patients required to compare different types of DES and has been accepted as a valid primary endpoint in head-to-head trials comparing different types of DES2. ISLL reflects the biological effect of DES (degree of inhibition of neo-intimal hyperplasia) in a very precise way. As ISLL is lowered the clinical benefit of DES (reduction in the need for new revascularisations) tends to increase2. In-segment late loss is also a valid quantitative parameter to measure the efficacy of DES, but it may be influenced by other factors such as vessel tapering, and edge effect, and thus ISLL reflects the biological effect of DES in a more precise way.
Methods
Design and inclusion/exclusion criteria
The CIBELES trial is a multicentre, controlled, single-blinded, randomised clinical trial (Clinicaltrials.gov number NCT00793221). It is planned to include 208 patients from 14 centres in Spain and Portugal. Patients ≥18 year-old with a total occlusion (Thrombolysis In Myocardial Infarction [TIMI] flow grade 0 or 1) with an estimated time of occlusion >2 weeks will be included. True chronic total occlusions are those with an estimated time since occlusion >3 months3. However, most randomised trials evaluating restenosis in CTO – mainly comparing BMS and balloon angioplasty- included patients with an estimated time since occlusion of >2 weeks, because in total coronary occlusions, estimated time since occlusion has more impact on the probability of obtain a successful recanalisation than on the risk of subsequent restenosis. Consequently, we decided to include patients with total coronary occlusions with an estimated time since occlusion of >2 weeks. In the PRISON-II trial, comparing SES with BMS in CTO, patients with an estimated time from occlusion >2 weeks were included.
Only patients with angina, silent ischaemia, or viable myocardium at the area supplied by the target vessel will be eligible for the trial. Exclusion criteria will be as follows: 1) Acute myocardial infarction at the area supplied by the target vessel within 2 weeks before the inclusion in the study; 2) The lesion can not be crossed with the guidewire and balloon angioplasty; 3) The target lesion has been previously treated percutaneously; 4) The lesion is not suitable for 2.25-3.5 mm coronary stent implantation; 5) The patient is not willing to undergo angiographic follow-up; 6) The patient has contraindications for prolonged double (aspirin plus thienopyridine) antiplatelet therapy (e.g., allergy to aspirin, need for chronic oral anti-coagulation, or scheduled major surgical intervention within 12 months); 7) Pregnancy or absence of a negative pregnancy test result in women of childbearing age; 8) Chronic renal failure (creatinine plasma value >3.0 mg/dl); 9) Plasma platelet count <100.000 mm–3 or >700.000 mm–3; 10) The patient has any severe non-cardiac disease that reduces his/her life expectancy to <1 year; 11) The patient is currently included in another randomised trial.
Randomisation will be performed on a 1:1 basis via a phone call to Chiltern International that will provide instructions on the allocated treatment. Randomisation will be confirmed by fax to the centre at which each patient is included. The study will be carried out under the rules and recommendations of ICH-GCP (CPMP/ICH/135/95), the Declaration of Helsinki, and the current Spanish and Portuguese legislation (Circular letter No. 07 /2004, RD 223/2004, RD 414/1996, UNE-EN ISO 14155-1, and UNE-EN ISO 14155-2). The ethical committees of all participating centres have revised and approved both the protocol and the informed consent before the beginning of the study. Every patient will give a written informed consent for the study before the procedure takes place.
Endpoints
The primary endpoint will be ISLL at 9-month angiographic follow-up. Any coronary angiography performed within four months after the initial procedure is considered unscheduled and will be invalid for the primary endpoint. Secondary angiographic endpoints include binary angiographic restenosis, vessel re-occlusion, and in-segment late loss, at nine months; Secondary clinical endpoints include death, myocardial infarction, new target vessel revascularisation, and angina status at one year.
Angiographic definitions
A total coronary artery occlusion is defined as the absence of antegrade flow of contrast distal to the occlusion (Thrombolysis and Myocardial Infarction [TIMI] flow 0 according to the TIMI score) or only minimal flow of contrast distal to the occluded vessel (TIMI flow 1). To be included in the study, the duration of the coronary occlusion has to be more than two weeks, estimated by clinical information, and angiographic findings. Chronic total coronary artery occlusion is defined as an occlusion with an estimated duration of >3 months3.
In-stent late loss is defined as the difference between minimum lumen diameter within the borders of the stent immediately after implantation, and that measured at angiographic follow-up (at nine months).
Binary angiographic restenosis is defined as the presence of >50% in-stent diameter stenosis, and vessel re-occlusion is defined as the presence of total vessel occlusion (100% diameter stenosis and TIMI flow 0 or 1) at angiographic follow-up.
Clinical definitions
An adverse event is every non desired event that occurs during the study, and it is not necessarily related to the drug or device under investigation. Serious adverse events include the appearance of any new disease, or worsening of previously existing diseases, or any laboratory abnormality that is considered clinically relevant by the investigators. Every adverse event occurring during the study should be registered in the clinical file and in the CRF. A serious adverse event is any adverse event leading to death or considered to be life-threatening, or with permanent sequelae or dysfunction, or leading to hospitalisation or a prolongation of the current hospitalisation.
The study has a clinical events committee including three cardiologists (two clinical cardiologists, and one interventional cardiologist) not participating in the trial and thus not involved in the treatment or follow-up of the patients. The three members of the events committee will be blinded to the type of DES implanted in each patient.
The investigators are required to inform the CRO about every serious cardiac event (death, myocardial infarction, stent thrombosis, or new target vessel revascularisation procedures), and vascular and bleeding complications within 48 hours of its diagnosis.
– Death. Deaths during the study will be classified as cardiac or non-cardiac by the events committee. All deaths will be considered as cardiac unless a clear non-cardiac cause of death can be identified.
– Acute myocardial infarction. The diagnosis of myocardial infarction will be defined as follows: 1) Q-wave myocardial infarction: the presence of new abnormal Q-waves, in association with a creatinine-phosphokinase (CPK-MB) elevation ≥2-fold the upper normal limit. 2) Non-Q-wave acute myocardial infarction: the presence of CPK-MB elevation ≥2-fold the upper normal limit associated with chest pain and/or ST-segment or T-waves abnormalities at the electrocardiogram.
– Stent thrombosis (ST). Episodes of stent thrombosis will be classified following the ARC (Academic Research Consortium) criteria17.
– New revascularisations. New target vessel revascularisations will only be considered clinically driven in presence of angina and ischaemia or myocardial viability demonstrated by non-invasive tests (treadmill test, myocardial scintigraphy, stress echocardiography) or invasive techniques (e.g., pressure guidewire). Any target vessel revascularisation will be considered target lesion revascularisation when located at the stented segment and/or the 5 mm adjacent to the stent.
– Vascular and bleeding complications: haematoma >5 cm, arterio-venous fistula, pseudoaneurysm and retroperitoneal bleeding will be considered as vascular complications. Bleeding or vascular complications will be considered severe when life-threatening or requiring surgical repair and/or blood transfusion.
Percutaneous coronary intervention (PCI) procedure
Unfractionated heparin will be administered i.v. during PCI, with additional bolus if necessary to maintain an activated clotting time (ACT) >300 seconds. The use of IIb/IIIa inhibitors will be left to the operator’s discretion, but in patients receiving these drugs, the initial dose of heparin (70 IU/kg), as well as the target ACT (200-250 seconds) will be lower. The use of enoxaparin or bivalirudin instead of unfractionated heparin during procedure will be allowed respecting local protocols.
The approach (radial vs. femoral), the type of guiding catheter, guidewire, and other technical issues (antegrade vs. retrograde approach, type of balloon, use of special devices such as rotational atherectomy, thrombus aspiration, cutting balloon, etc.) will be left to the operator’s preferences and local clinical practice.
It is recommended that the implanted coronary stent cover entirely the segment dilated by the balloon. The use of additional coronary stents will be allowed if necessary to entirely cover the lesion. If in-stent residual stenosis is >20% by visual estimation after coronary stent implantation, post-dilation is recommended.
Follow-up
A 12-lead electrocardiogram and blood sample, including determination of cardiac enzymes (troponin, or creatinine-phosphokinase MB), creatinine, and haemoglobin, will be taken 24 hours after PCI. A complete clinical evaluation will be performed at discharge to record myocardial infarction, target vessel revascularisation, stroke, ST and major and minor bleeding. The same clinical endpoints, as well as functional status for angina (classification of the Canadian Society of Cardiology) will also be evaluated at one month, and one year after PCI.
As a recommendation, thienopyridines will be withdrawn the day after angiographic follow-up, unless the patient would need a subsequent PCI. Angiographic follow-up will be scheduled at nine months after PCI. In the presence of binary angiographic restenosis, a new percutaneous target vessel revascularisation will be allowed if clinically indicated (angina, silent ischaemia or myocardial viability) and technically possible. In patients with binary angiographic restenosis and a clinical indication of new revascularisation, coronary artery bypass grafting can be considered if the restenosis is not suitable for a repeated PCI.
Angiographic analysis
Quantitative coronary analysis will be performed at a central core lab (Hospital Clinico San Carlos, Madrid, Spain), by experienced physicians, blinded to the type of stent implanted, and not involved in the treatment of the patients.
Two orthogonal views will be selected to completely and correctly visualise the target lesion, trying to avoid vessel overlapping and angiographic shortening of the vessel. The target lesion will be maintained in the centre of the screen, avoiding panning.
The two best angiographic views will be used for the immediate angiographic results, as well as for the angiographic follow-up. All the images will be obtained after administration of nitroglycerin 0.2 mg i.c. The MEDIS QCA software (MEDIS, Leiden, The Netherlands) will be used for QCA. The following angiographic variables will be measured:
– Reference vessel diameter (mm), minimum lumen diameter (mm), percentage of stenosis (%), and lesion length: at baseline, immediately after PCI, and at angiographic follow-up.
– TIMI grade flow: at baseline, immediately after PCI, and at angiographic follow-up. This classification establishes 4 grades of coronary flow: 0 (absence of antegrade flow); 1 (antegrade flow, but without distal filling of the vessel); 2 (antegrade flow, with distal vessel filling, but slow in comparison with other epicardial vessels); and 3 (antegrade flow, with distal vessel filling not slow in comparison with other epicardial vessels).
– Collateral circulation to the occluded vessel following the Rentrop´s classification: 1) Grade 0: no visualisation of collaterals; 2) Grade 1: poor visualisation of collaterals to the occluded vessel; 3) Grade 2: visualisation of collateral vessels with partial epicardial filling of the occluded vessel; 4) Grade 3: visualisation of collaterals with complete epicardial filling of the occluded vessel.
The estimated length of the occlusion will be measured from the point of the total occlusion to the most proximal point of the distal vessel, when visualised by collateral filling with contrast. The total coronary analysis segment is defined as the stented segment including the margins 5 mm distal and proximal to the stents.
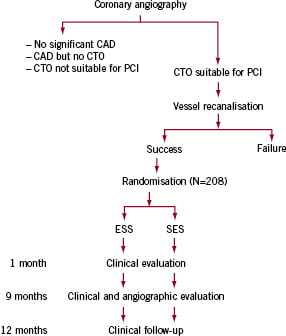
Figure 1. Flow chart of the screening and inclusion of the patients in the CIBELES trial. Only patients with successful recanalisation of the CTO will be included.
Sample size calculation
In the SPIRIT-I and II trials, mean ISLL for EES during follow-up was 0.10, and 0.11 mm respectively. For SES, different studies have shown ISLL ranging from 0.05 to 0.28 mm2. Therefore, it will be assumed that both EES and SES have the same mean values of ISLL, and a standard deviation of 0.45 mm will also be assumed for both groups. The estimated number of patients per group needed to demonstrate the non-inferiority of EES vs. SES in terms of ISLL (non-inferiority margin 0.20 mm), with a one-sided α error of 0.05, and β error of 0.10 (90% statistical power) is 88. If we estimate that 15% of patients will not finally undergo angiographic follow-up, 104 patients would need to be included in each group. Thus, the total number of patients to be included is 208. In order to assure that at least 50% of patients included will have an estimated time since occlusion of >3 months, the inclusion of patients with an estimated time since occlusion from two weeks to three months will be stopped at 104.
Statistical analysis
Statistical analysis will be performed with the SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, USA). The primary endpoint (in-stent late loss) will be assessed by a one-sided test for non-inferiority using the Student´s t-test (comparison of a continuous variable in two groups). Discrete variables will be compared with the χ2 test (Fisher´s correction when necessary). Differences will be considered statistically significant when p<0.05, although all p values will be provided.
Conclusions
CTOs constitute one of the scenarios in which DES may offer an angiographic and clinical benefit in comparison with BMS. The use of new DES is being increasingly seen in daily clinical practice. The CIBELES trial will demonstrate if the second-generation DES EES is comparable to SES in this angiographic setting.

