Abstract
Aims: We sought to assess the safety and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold (BVS) in percutaneous chronic total occlusion (CTO) revascularisation guided by intracoronary imaging. The feasibility of using the BVS in CTO lesions is unknown.
Methods and results: Thirty-five consecutive true CTO lesions (EuroCTO Club definition) were included in this prospective registry. After mandatory predilatation and IVUS analysis, all target lesions were treated with BVS and no other stents were deployed. Optical coherence tomography (OCT) was performed after BVS implantation. Multislice computed tomography (MSCT) was performed at baseline and at six to eight months. The mean age was 60.7±9.7 years; 80% were male; 20% were diabetic; 37% had a previous PCI. The most frequently treated vessel was the RCA (46%). According to the Japanese-CTO (J-CTO) complexity score, most lesions were classified as intermediate (49%) or difficult-very difficult (26%); 34% were moderate-severely calcified. Most cases (86%) were treated with an anterograde strategy, 60% by radial or biradial approach. In 71% a cutting balloon was used. The total scaffold length implanted per lesion was 52.5±22.9 mm. All scaffolds were successfully delivered and deployed. Post-dilatation was undertaken in 63%. By OCT, final minimum scaffold area and lumen stenosis were 7.1±1.5 mm2 and 11.7±6.6%, without areas of significant strut malapposition. At complete six-month follow-up, no major adverse events were observed. MSCT identified two cases of scaffold reocclusion.
Conclusions: BVS for CTO recanalisation demonstrates excellent feasibility and safety as well as midterm efficacy. Appropriate lesion preparation is key to aiding adequate expansion of these scaffolds in this setting.
Introduction
Recanalisation of chronic total occlusions (CTO) is one of the most challenging percutaneous coronary interventions (PCI). Procedural success is hampered by the difficulties associated with crossing the occluded segment with guidewires and recanalisation devices. Long lengths of stent are often required and long-term results are threatened by a high restenosis rate1. The introduction of drug-eluting stents (DES) raised hopes of improving long-term vessel patency after CTO recanalisation2. However, very late stent thrombosis is a rare and dangerous complication that might be associated with the off-label use of DES3,4.
The everolimus-eluting bioresorbable vascular scaffold (BVS) represents a promising new technology with some potential benefits. The term “scaffold” is used to describe a temporary bioresorbable platform which provides transient vessel support to resist acute recoil, with drug delivery capability, and at a later stage (two to three years) full resorption occurs, without the long-term limitations of metallic DES5. Therefore, in the long term the BVS potentially:
– restores more normal vascular physiology of the treated vessel
– permits vascular remodelling and leads to late lumen enlargement
– “liberates” jailed side branches and solves problems of malapposition
– permits future surgical revascularisation if needed
Furthermore, BVS are invisible when imaging the coronary arteries non-invasively, such as with multislice computed tomography6. Despite all those potential advantages, there is as yet no evidence supporting the use of BVS in CTO. Questions have also been raised concerning the delivery of the scaffold to complex lesions7.
Methods
PATIENT POPULATION
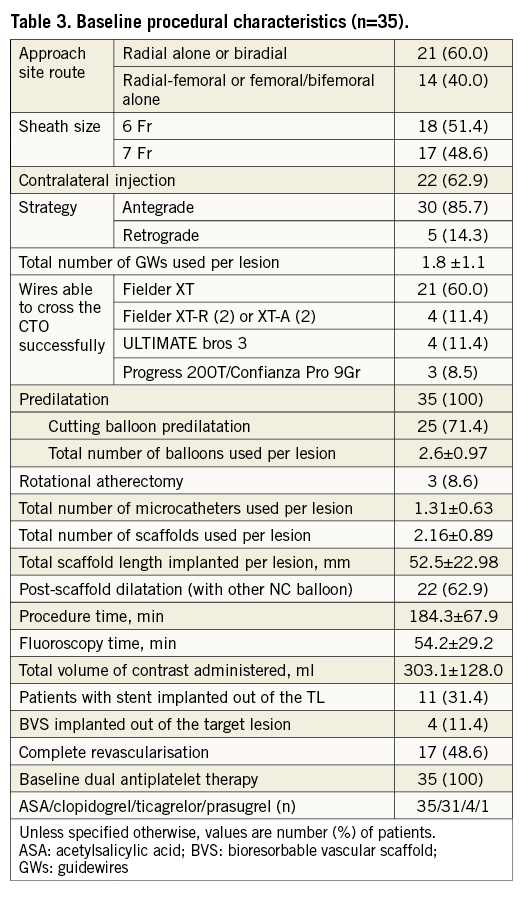
The ABSORB-CTO pilot study is a single-centre trial, assessing the safety and performance of the Absorb BVS (Abbott Vascular, Santa Clara, CA, USA) in the treatment of patients with at least one true CTO (EuroCTO Club definition)8, with a reference vessel diameter between 2.5 and 3.5 mm. Major exclusion criteria were: lesions located in the left main coronary artery, lesions involving a true bifurcation lesion with a side branch ≥2.5 mm in diameter by visual assessment, the presence of a contraindication to drug-eluting stent implantation, and lesions placed in vessels with a reference diameter <2.5 or ≥4.0 mm. Angiographic target lesion calcification was not a predefined exclusion criterion, and left to the operator to decide. As a general rule, only resistant or non-dilatable calcified lesions after plaque modification (cutting balloon and/or rotational atherectomy) and confirmation by IVUS evaluation were excluded. From 49 clinically eligible CTO patients, 14 patients (28.5%) were excluded, due to the predefined angiographic criteria (n=11) or because of procedural complications needing bail-out stent implantation (n=3) (Figure 1). From February 2013 until March 2014, 33 patients (35 CTO lesions) were thus enrolled in the ABSORB-CTO pilot study.
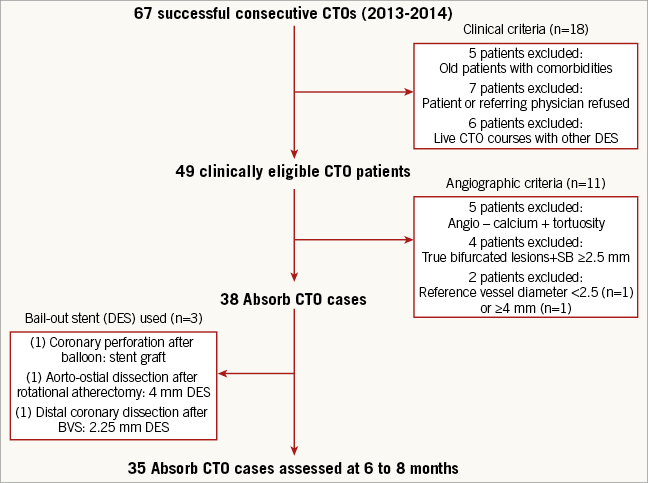
Figure 1. Study profile.
The study was approved by the local ethics committee and all patients provided written informed consent.
STUDY DEVICE
The second-generation Absorb BVS consists of a polymer backbone of poly-L-lactide (PLLA) coated with a thin layer of a 1:1 mixture of poly-D,L-lactide (PDLLA) polymer. The PDLLA coating contains and controls the release of the antiproliferative drug, 100 µg of everolimus/cm2 of scaffold. Both PLLA and PDLLA are fully bioresorbable and degrade to lactic acid, which is metabolised via the Krebs cycle. The Absorb BVS has struts with a thickness of 157 µm and zigzag hoops connected by three links, similar to the XIENCE V design (Abbott Vascular). The details of the device have been previously described9.
INTERVENTIONAL PROCEDURE AND LESION PREPARATION (Figure 2)
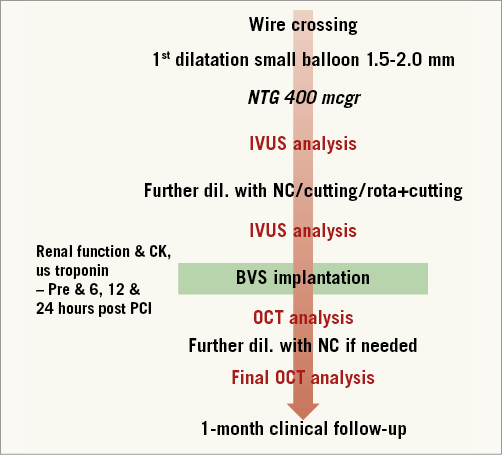
Figure 2. Flow chart of the study.
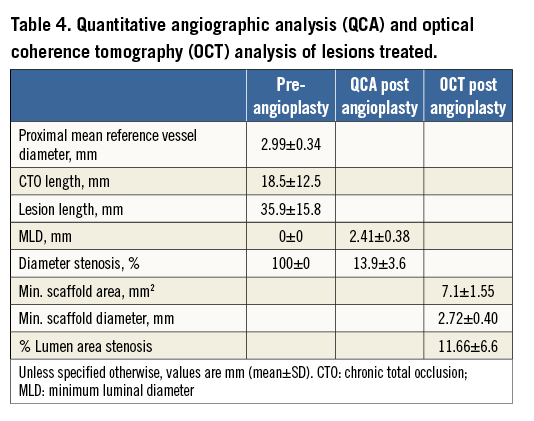
The PCI was performed as an elective procedure. In our CTO programme, prior to the procedure multislice computed tomography (MSCT), cardiac magnetic resonance imaging (MRI), quality of life and six-minute walk test are recommended for all patients. After detailed clinical assessment, including review of angiographic images and MSCT data, the PCI strategy (contralateral injections, guiding catheter choice, “soft” or “hard” approach, anterograde or retrograde approach) was decided for each case. Patients were pre-treated with dual antiplatelet therapy. During the procedure, activated clotting time was maintained above 250 sec. After crossing the occlusion with a guidewire, dilatation was undertaken initially with small balloons (1.5 or 2.0 mm). After nitroglycerine (up to 600 mcg), vessel size and lesion length were determined by quantitative coronary angiography (QCA) (Figure 3). Accordingly, the correct balloon size was used to predilate the entire lesion. Before scaffold implantation, intravascular ultrasound (IVUS) with a 40 MHz transducer was performed to analyse the morphological and anatomical characteristics of the lesion (fibrocalcific or calcified plaque not apparent angiographically, areas of inadequate expansion) (Figure 4). Predilatation of the entire lesion was optimised by either cutting or non-compliant (NC) balloons (ratio balloon/artery 0.7-1:1) to minimise the risk of underexpansion of the scaffold in any area of the entire plaque. The result of this optimisation was verified again by IVUS.
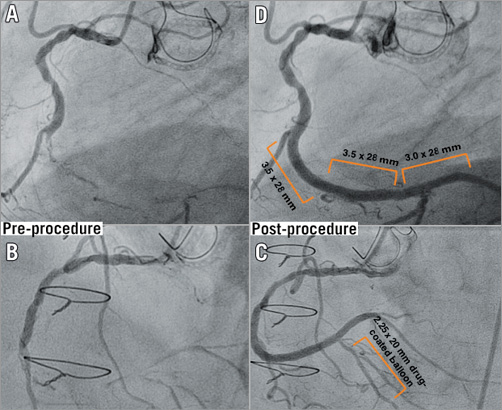
Figure 3. Serial angiograms before and after the procedure. From A to C, angiogram of the mid distal right coronary artery (RCA), CTO lesion, before procedure, and after procedure. It was a really long lesion that involved the distal and mid RCA segments and distal bifurcated lesion (posterolateral branch [PL]-posterior descending [PD] branch) (A and B). The PD branch was treated by drug-eluting balloon technology (C) and then, after careful plaque modification, three overlapped Absorb BVS were successfully implanted from distal (PL branch) to proximal (mid segment of the RCA) (D).
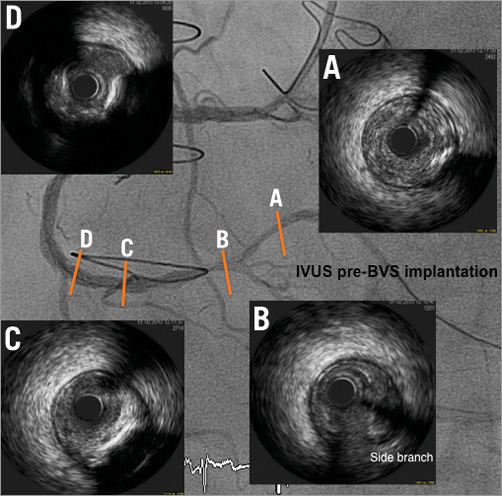
Figure 4. Four IVUS cross-sectional areas (A-D) identified and localised on the RCA coronary angiogram after small balloon predilatation. There was a moderate calcified segment in the mid RCA which was predilated with a 3.5 mm scoring balloon before BVS implantation.
If the predilatation was considered optimal, BVS was selected according to IVUS measurements and implanted following the instructions for use: increasing pressure by 2 atm every five seconds up to 12-14 atm, overlapping by only 1-2 mm, and re-dilatation if needed with a shorter, NC balloon at nominal pressure with a maximal increase of the balloon above scaffold size of 0.5 mm. When acute procedural success was achieved (final in-scaffold residual stenosis of <50% and TIMI 3 flow, without any complication), coronary angiography in two orthogonal projections was performed for QCA analysis. Finally, an OCT study of the entire treated segment was undertaken with high-pressure NC balloon post-dilatation for any residual stenosis of more than 20% (Figure 5).
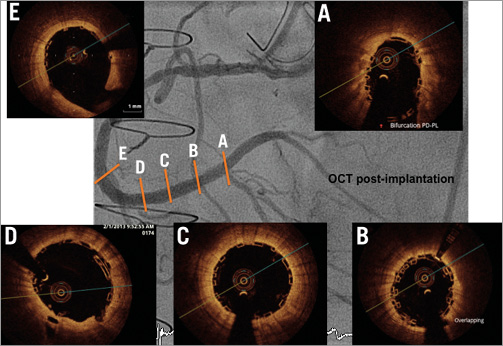
Figure 5. Five optical coherence tomography (OCT) cross-sectional areas (A-E) identified and localised on the RCA coronary angiogram. Stent struts imaged by optical coherence tomography have the appearance of a black box. OCT demonstrated post-procedural good deployment and apposition.
STUDY DEFINITIONS
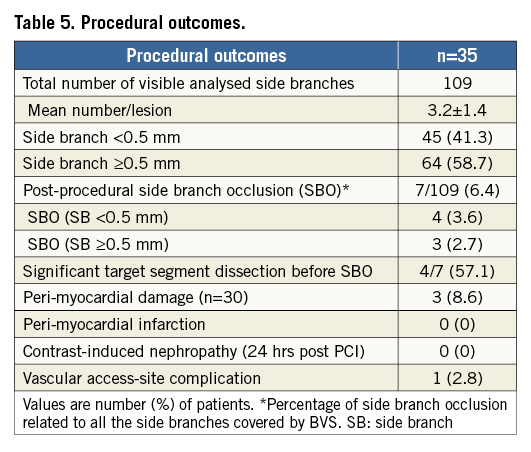
Acute procedural success was defined as achievement of final in-scaffold residual stenosis of <30% (by QCA and confirmed by OCT) and TIMI 3 flow. Acute device success was defined as successful delivery and deployment of the study scaffold(s) to cover the intended target lesion and withdrawal of the delivery system, attainment of <30% residual stenosis and TIMI 3 flow without the need for other non-study stents. Scaffold thrombosis was defined using Academic Research Consortium (ARC) definitions for definite or probable stent thrombosis at the time of intervention and at follow-up. Deaths from undetermined causes were classified as cardiac. Periprocedural myocardial damage was defined as creatine kinase (CK) elevation ≥ three times the upper limit of normal (ULN) accompanied by an increased level of us troponin elevation ≥5 x ULN. Enzyme elevation in the presence of symptoms and/or electrocardiogram changes suggesting MI was defined as periprocedural myocardial infarction (MI)10. During follow-up, MI was defined as CK elevation ≥3 x ULN with us troponin elevation ≥5 x ULN. In that case, per protocol, enzymes were only measured in case of symptoms and/or electrocardiogram changes suggesting MI. Target lesion revascularisation (TLR) was ischaemia-driven and included the scaffold segment and 5 mm proximal and distal beyond the scaffold edges. Major adverse cardiac events (MACE) were defined as the composite of ischaemia-driven target lesion revascularisation (ID-TLR), myocardial infarction and cardiac death. Target vessel revascularisation (TVR), also ischaemia-driven, was defined as repeat revascularisation within the treated vessel. Procedure-related contrast-induced nephropathy (CIN) was defined as an increase of 25% or 0.5 mg/dl in serum creatinine at 24 to 48 hours after PCI comparing baseline values11. Side branch occlusion (SBO) was defined as a reduction in TIMI flow to grade 0 or 1. Accordingly, side branches with pre-BVS implantation TIMI flow grade 0 or 1 were excluded12.
ANGIOGRAPHIC ASSESSMENT
QCA analysis was performed according to standard procedures13, using dedicated software (Medis QAngio XA 7.3 software; Medis medical imaging systems bv, Leiden, The Netherands). In each patient, the treated and peri-scaffold segments (defined as 5 mm proximal and distal to the scaffold edge) were analysed by QCA, in paired matched angiographic views before and after the procedure. Small radiopaque markers (37 μm) at the ends of the stents helped with the localisation of the non-radiopaque stent for QCA. The following QCA parameters were computed: minimal luminal diameter (MLD) and area, and reference vessel diameter (obtained by interpolation of the expected dimensions of the coronary vessel at the point of obstruction). Percentage diameter stenosis was subsequently ascertained in scaffold, in peri-scaffold segment, and in segment (scaffold plus peri-scaffold segments)8. At baseline, the values for diameter stenosis and minimal lumen diameter (MLD) were 100% and 0 mm, respectively, in all patients. Angiographic success was defined as less than 30% final residual stenosis in the treated segment and the absence of more than type B coronary dissection post BVS angioplasty.
OPTICAL COHERENCE TOMOGRAPHY (OCT) ANALYSIS
Dragonfly frequency-domain optical coherence tomography (FD-OCT) (C7-XR system; St. Jude Medical, St. Paul, MN, USA) was performed after the procedure. The OCT measurements were performed using dedicated software (Medis QIvus 3.0 with OCT quantification software 3.0; Medis medical imaging systems bv). BVS is very different from metallic stents when imaged by OCT8. At baseline, the scaffold area is identical to the lumen area in the absence of incomplete scaffold apposition (ISA) and prolapse. The lumen and scaffold contours were obtained with a semi-automated detection algorithm available in the Medis QIvus 3.0 with OCT quantification software 3.0. The 3D reconstruction was performed with QAngio OCT Research v1.0 software (Medis). The treated and same peri-scaffold segments were analysed as per QCA. Post BVS implantation, the following quantitative parameters were registered: mean and minimal lumen and scaffold area, lumen and scaffold residual stenosis (%) and incomplete scaffold apposition (ISA) area. Qualitatively, the diagnosis of procedural strut fracture resulting from balloon overinflation was made if two struts overhung each other in the same sector of the lumen perimeter, with or without malapposition, or if isolated struts were located towards the centre of the vessel without obvious connection with surrounding struts8. To confirm the diagnosis, it was helpful to perform 3D OCT reconstruction of the disrupted strut.
MULTISLICE CT CORONARY ANGIOGRAPHY
A 256-detector Philips Brilliance iCT system (Brilliance iCT; Philips Healthcare, Cleveland, OH, USA) was used. Standard acquisition techniques were employed, including beta-blockers (5 to 20 mg of intravenous metoprolol) in patients with a heart rate >65 bpm and 0.4 mg of sublingual nitroglycerine in all cases. Axial scan protocols for patients with lower (<65 bpm) heart rates (acquisition using “step-and-shoot” protocol) were performed to reduce radiation dose. Patients received 90 mL (when <75 kg), 100 mL (between 75 and 90 kg) or 110 mL (when >90 kg) of iodinated contrast (Optiray 350; Mallinckrodt Medical Imaging, Dublin, Ireland) at an infusion rate of 6 mL/s followed by a 40 mL saline flush at 4 mL/s. Using a bolus tracking technique, acquisition started when a threshold of 200 Hounsfield units (HU) was reached in the descending aorta. Images were reconstructed using thin slices (0.4 to 0.5 mm).
FOLLOW-UP PROCEDURES
Blood tests for renal function, CK and us troponin were performed before and at six, 12 and 24 hours post procedure. Clinical follow-up was scheduled at one, six, 12, 18, and 24 months. An MSCT scan was planned in all patients at six months (Figure 6).
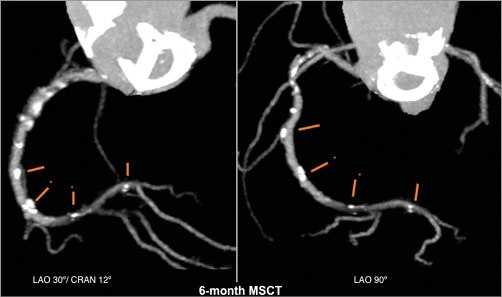
Figure 6. Multislice CT angiography at six months. This delineates easily the contours of the stented vessel since the stent struts are not radiopaque, showing that the scaffolded segment was patent.
STATISTICAL ANALYSIS
Continuous variables are presented as mean and standard deviation and categorical variables as number and percentage. Statistical analysis was performed using commercially available software (IBM SPSS 21 for Windows; IBM Corp, Armonk, NY, USA).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
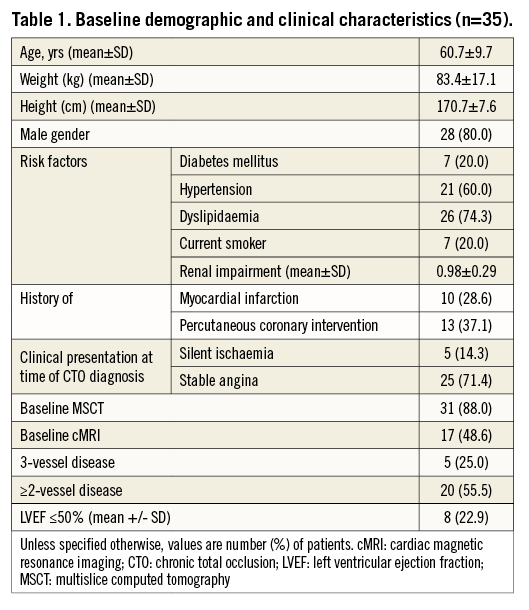
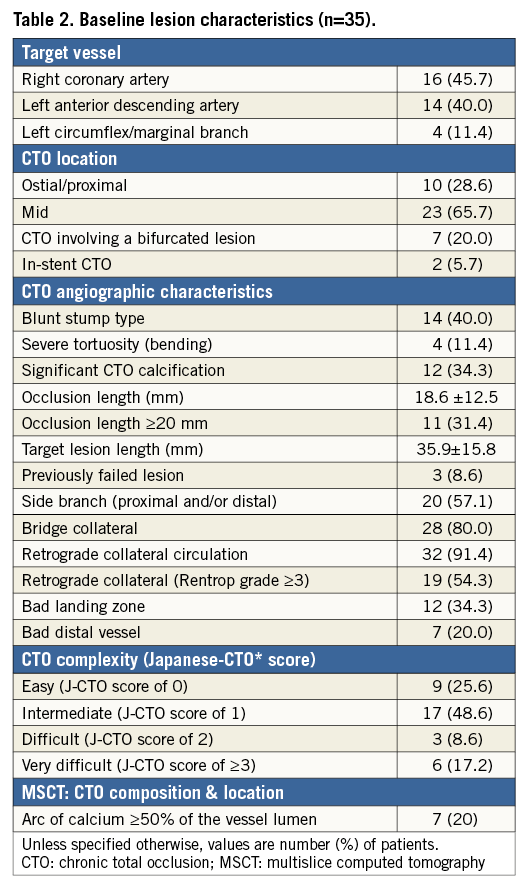
A total of 35 CTO lesions were prospectively included in the study. The population was relatively young (Table 1) with a mean age of 61±0 years; 37% had a previous PCI. Most of the patients (86%) were stable at presentation, and baseline MRI was performed in almost half of the population. In 88% of cases, an MSCT was performed before the procedure. Table 2 shows baseline lesion characteristics. The CTO complexity score was determined before the procedure in all cases, according to the J-CTO (Multicenter CTO Registry of Japan) score14. Most of the lesions were classified as intermediate (49%) or difficult-very difficult (26%); 34% were moderate-severely calcified lesions. By MSCT, 20% of CTO lesions had an arc of calcium between 50 and 75% of the lumen. Concerning the procedure (Table 3), predilatation was undertaken in all cases, by cutting balloon in 71% and rotablator in 8.5% of the cases. In most cases (86%), the strategy was antegrade. Fifty-one percent were performed with a 6 Fr catheter and in 60% of the cases the radial or biradial approach was used. The total scaffold length implanted per lesion was 52.5±22.8 mm. Post-dilatation was undertaken in 63%. Reference vessel diameter was 2.9±0.34 mm and final diameter stenosis and acute gain were 12.1±5.5% and 2.56±0.37 mm, respectively (Table 4).
SHORT AND MIDTERM OUTCOMES WITH MSCT
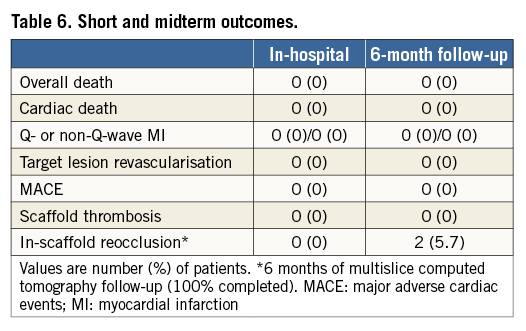
The procedural and midterm outcomes are summarised in Table 5 and Table 6. All scaffolds were delivered and deployed successfully without any significant scaffold malapposition by final OCT analysis. According to our definitions, we report three cases of myocardial damage and no MI. These cases were technically complex, two having been performed by a retrograde approach. In these cases, side branch occlusion (SBO) was not related to enzyme elevation. SBO was observed in 6.4% (7/109) of visible side branches covered by BVS (three LAD, two RCA and two circumflex arteries). Smaller side branches (SB <0.5 mm) were more frequently occluded (3.6%) than SB ≥0.5 mm (2.7%). In all three cases of larger SBO (SB ≥0.5 mm), a significant dissection after balloon dilatation and before BVS implantation was observed. We also report a significant femoral occlusion after 24 hours of the index procedure that was treated by stent. The case in question was a complex, retrograde case using an 8 Fr femoral sheath. We report no contrast-induced nephropathy. Clinical follow-up was available for all patients at one month and no MACE was reported. The cumulative rate of adverse events at six months is given in Table 6. MSCT was performed and completed in all cases. We report two cases of scaffold reocclusion, which were confirmed by invasive angiography.
Discussion
The present study is the first demonstrating the feasibility, safety and short-term efficacy of PCI for CTO lesions with the Absorb BVS. The main findings of the ABSORB-CTO pilot study are the following. 1) PCI for consecutive and non-selected patients with true CTO lesions, using multiple BVS (mean scaffold length 53 mm), is feasible with all the scaffolds delivered and deployed successfully. 2) Post-procedural SBO after BVS implantation was observed in 6.4% of all visible side branches. 3) At one-month follow-up no major adverse events were observed. 4) Finally, these results were achieved thanks to an appropriate lesion preparation assuring adequate expansion of fully bioresorbable scaffolds in this setting. IVUS guidance could be useful in some complex and calcified CTO cases, to size the vessel appropriately and avoid unexpected scaffold underexpansion.
POTENTIAL BENEFITS OF ABSORB BVS FOR THE TREATMENT OF CTO LESIONS
According to the European Registry of CTOs (ERCTO) we present a physically active population with long lesions necessitating the use of more than one stent (mean scaffold length per lesion 53 mm)15. In such lesions, the risk of delayed endothelialisation with DES in combination with suboptimally implanted DES may be associated with a higher and longer-term risk of stent thrombosis. Furthermore, in this setting, the traditional “full metal jacket” may jail some significant side branches and in some cases may preclude future surgical revascularisation. In this specific setting, the polymeric material as an implantation medium has numerous potential long-term advantages compared to metal. First, a fully bioresorbable scaffold might have less potential for late stent thrombosis because of the elimination of late acquired or persistent malapposition and the risk of delayed or incomplete endothelialisation16. Second, with the potential integration into the vessel wall of the polymeric struts, jailed side branches will be freed. The absence of permanent metal also facilitates reinterventions. Finally, the absence of a permanent metal endoluminal prosthesis should allow the lumen to change dynamically in response to physiological or pharmacological stimuli17.
Despite all these potential benefits, to date the treatment of coronary artery disease with the Absorb BVS has been investigated in a limited number of patients with relatively simple coronary lesions6,9,12,17. The results of the ABSORB II randomised trial, comparing the metallic everolimus-eluting stent XIENCE with the Absorb BVS and including small vessels and long lesions, is awaited18. Meanwhile, our ABSORB-CTO pilot study demonstrates feasibility and short-term safety and, when comparing historically, seems to show similar efficacy to the best DES. Despite these satisfactory midterm results in 70% of CTO lesions, concerns remain regarding use of this device for such lesions, which may be the substrate for long segments of late negative remodelling potentially limiting long-term durability19.
USE OF BVS IN CLINICAL PRACTICE
The use of BVS in coronary interventions other than in controlled studies is increasing. Questions have been raised concerning the delivery and deployment of the scaffold to complex lesions. The potential limitations to the use of the Absorb BVS result from thicker struts (currently 157 μm in comparison to around 70-85 μm with current-generation DES), limited distensibility with risk of scaffold fracture, and limited device sizes (diameter and length) in comparison to DES. Regarding deliverability and deployment in our study, all lesions were predilated (2.6 balloons used per lesion), using cutting balloons in 71% of the cases. In addition, our protocol mandated IVUS guidance to verify adequate lesion preparation to facilitate scaffold expansion.
The greater strut thickness of the Absorb BVS compared to current DES also warrants consideration in terms of the vessel wall area covered and the resulting increased probability of covering the orifice of side branches12. In our study including long lesions covered by BVS (109 visible SB analysed in 35 CTO cases), where the incidence of SBO might have been expected to be higher, we report a relatively low rate of SBO (6.4%) after BVS implantation. This rate of SBO was similar to that observed in the post hoc angiographic assessment of patients enrolled in the ABSORB EXTEND trial12. In keeping with the results of this trial, very small side branches (<0.5 mm) occluded more often, 3.6% versus 2.7% (SB ≥0.5 mm). In our trial, it was also noted that there was significant SB dissection after balloon dilatation and so, in fact, occlusion was probably unrelated to BVS implantation in 57% of cases, including all three cases of larger SBO (SB ≥0.5 mm). In our experience, this is something to consider when treating lesions involving long segments of the left anterior descending artery, with several small septal branches. Further investigation is required in a randomised trial to clarify this issue.
Regarding the limited expansion of the BVS, although the radial strength of the Absorb has been reported to be comparable to metallic stents, this is only when the BVS is deployed within the limits of its size20. In our IVUS-guided study, care was taken to match devices appropriately to vessel size after thorough predilatation. On post-procedural OCT, we did not report any scaffold fracture. In 63% of cases, NC balloon post-dilatation was performed using a shorter and 0.5 mm larger balloon compared to the implanted BVS without exceeding the expansion limits of the scaffold. Thus, keeping in mind proper vessel sizing, lesion preparation and scaffold expansion limits, PCI for CTO lesions using multiple BVS is feasible, as it was in our study with all the scaffolds delivered and deployed successfully.
USEFULNESS OF MULTISLICE CT ANGIOGRAPHY TO ASSESS ABSORB BVS
We used multislice CT to assess the midterm results at six months. Patency and absence were established non-invasively. In a subset of patients previously reported and investigated after the BVS procedure, the quantitative results of 2D and 3D QCA conventional angiography, 3D intravascular ultrasound, and MSCT angiography were positively correlated from immediately after the procedure to six months21. The feasibility and accuracy of the use of multislice CT in the analysis of radiolucent biodegradable stents in a multicentre study could indicate a new era for non-invasive assessment of patients treated with radiolucent stents. We found that one of the limitations of MSCT to assess BVS was the small size of radiomarkers (37 μm) at the ends of the radiolucent scaffold. These platinum markers facilitate the accurate positioning and deployment of the device, but render the assessment of the patency by non-invasive MSCT problematic in the overlapped areas. So far, the clinical validation of this new methodological approach has only been obtained in one clinical trial and awaits further validation21.
LIMITATION OF THE STUDY
This study is not randomised with a relatively small sample size because it was designed to provide preliminary observations and generate hypotheses for future larger studies. The follow-up might be too short to capture all the relevant events, and we will undertake further invasive and non-invasive follow-up.
Conclusion
The ABSORB-CTO pilot study has demonstrated the feasibility of PCI of chronic total coronary occlusions with the fully bioresorbable Absorb BVS. We have demonstrated midterm safety and efficacy. The use of the Absorb BVS might prove to be useful, especially in long segments of disease, to avoid “full metal jackets” in order to allow possible future treatments such as CABG, and where the benefit of a restored functional endothelium might be most beneficial. Further clinical and imaging follow-up at future time points is required to extend the utility of the current findings.
| Impact on daily practice The potential benefits of the bioresorbable technology have been shown in the ABSORB first-in-man trials with long-term follow-up and several imaging modalities. However, questions have been raised concerning the delivery and deployment of the scaffold to complex lesions. The present study is the first to demonstrate feasibility, safety and midterm efficacy of CTO percutaneous revascularisation using the Absorb BVS technology. This registry has provided preliminary observations suggesting that, in the most complex scenario, bioresorbable scaffolds can deliver results similar to second-generation DES with the appropriate implantation technique (appropriate lesion preparation). We will need to confirm these promising results in future larger studies with further long-term follow-up. |
Acknowledgements
The authors wish to express their sincere appreciation to Angeles Mañas, nurse from Hospital de la Santa Creu y Sant Pau, Barcelona, for her support in data collection. The authors would like to thank Dr Helen Routledge, MD, FRCP, for her valuable comments and review of the manuscript.
Funding
This study received financial support from Abbott Vascular, USA. The sponsor has no role in the design of the study, data collection, data analysis, data interpretation or writing of the report.
Conflict of interest statement
The authors have no conflicts of interest to declare.

