
Abstract
Aims: Bioresorbable vascular scaffolds (BVS) represent a novel therapeutic option for the treatment of coronary artery diseases. The objective of this study was to evaluate the feasibility of BVS implantation in complex chronic total occlusions (CTO).
Methods and results: The present report is a multicentre registry evaluating results after BVS deployment in challenging CTO lesions, defined as J-CTO score ≥2 (difficult or very difficult). A total of 105 patients were included in the present analysis. The mean J-CTO score was 2.61 (difficult 52.4%, very difficult 47.6%). Device success and procedural success rates were 98.1% and 97.1%, respectively. The retrograde approach was used in 25.7% of cases. After wire crossing, predilatation was performed in all cases with a mean predilatation balloon diameter of 2.73±0.43 mm. The mean scaffold length was 59.75±25.85 mm, with post-dilatation performed in 89.5% of the cases and a mean post-dilatation balloon diameter of 3.35±0.44 mm. Post-PCI minimal lumen diameter was 2.50±0.51 mm and percentage diameter stenosis 14.53±10.31%. At six-month follow-up, a total of three events were reported: one periprocedural myocardial infarction, one late scaffold thrombosis and one additional target lesion revascularisation.
Conclusions: The present report suggests the feasibility of BVS implantation in complex CTO lesions, given adequate lesion preparation and post-dilatation, with good acute angiographic results and midterm clinical outcomes.
Abbreviations
BVS: bioresorbable vascular scaffolds
CTO: chronic total occlusions
ISA: incomplete strut apposition
MACE: major adverse cardiac events
MI: myocardial infarction
MLD: minimal lumen diameter
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
RVD: reference vessel diameter
ST: scaffold thrombosis
TLR: target lesion revascularisation
%DS: percentage diameter stenosis
Introduction
Recanalisation of chronic total occlusions (CTO) by percutaneous coronary intervention (PCI) is associated with angina relief, improved left ventricular function1-3, reduction in the rate of myocardial infarction and coronary artery bypass grafting with possibly improved patient survival4-9, even in patients with well-developed collateral circulation9.
However, despite recent advances in techniques and materials, the success rate for PCI in CTO is significantly lower compared to other lesion subsets10,11. CTO is present in up to 20% of patients with coronary artery disease undergoing elective angiography12,13, yet this lesion subset represents only a minority of the target lesions treated with PCI13,14. In addition, CTO are often long lesions requiring the implantation of multiple metallic stents resulting in a “full metal jacket” treated artery15, potentially increasing the risk of thrombosis and restenosis16.
Furthermore, many patients with CTO have extensive coronary disease, requiring multivessel revascularisation7,17, potentially benefiting from future bypass graft surgery. Given this background, the introduction of bioresorbable vascular scaffolds (BVS) provides a novel therapeutic option for the treatment of chronic total occlusions, allowing revascularisation without permanent caging of long coronary segments, potentially reducing the long-term limitations related to the presence of metallic stents and maintaining the option for future surgical revascularisations.
Bioresorbable technologies have so far mostly been tested in relatively simple coronary lesions18,19, but there are very limited data and experience with BVS in complex scenarios, especially in CTO lesions20-22.
Therefore, the objective of the present study was to evaluate the feasibility and midterm performance of BVS in CTO with challenging characteristics according to the J-CTO score23.
Methods
This was a multicentre single-arm study conducted in the Netherlands, England, Italy, Spain and India from March 2015 to March 2016, to evaluate the feasibility, procedural results and midterm performance of the Absorb™ BVS (Abbott Vascular, Santa Clara, CA, USA) in patients with complex CTO lesions (Figure 1).
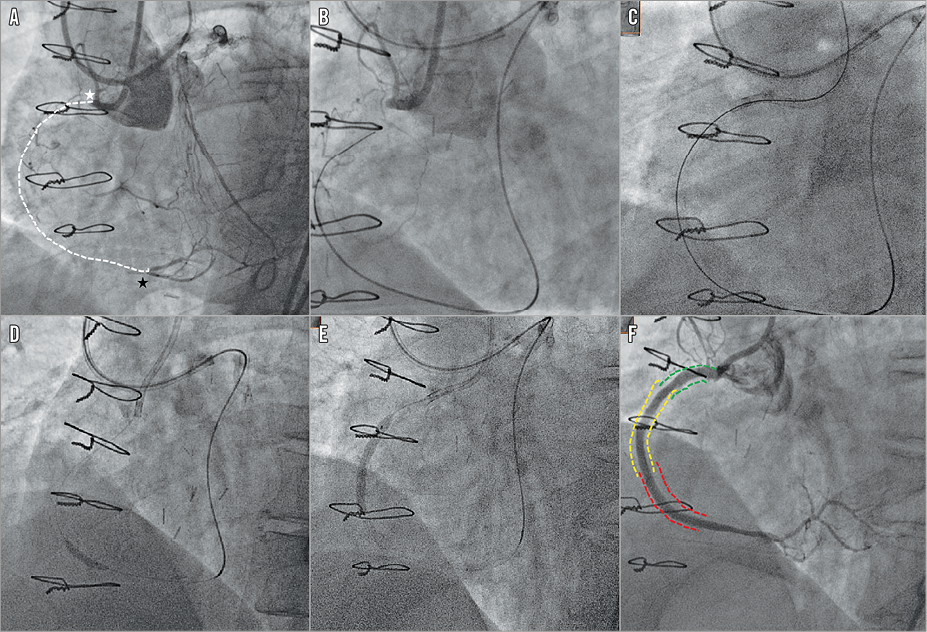
Figure 1. Successful implantation of bioresorbable vascular scaffolds in a right coronary artery with a chronic total occlusion using a retrograde strategy. The lesion had a blunt stump (white star) with a good distal landing zone (black star) (A). Good septal collaterals were seen from contralateral contrast injection. Successful crossing was achieved with a Conquest Pro wire on a Corsair microcatheter support (Asahi Intecc) (B & C). Predilation (D) was performed before scaffold deployment (two BVS 3.5×28 mm and one 3.0×28 mm in overlap) (E), with a good angiographic result (F).
Enrolled patients presented with silent ischaemia, stable angina pectoris or acute coronary syndromes. Lesion difficulty was graded according to the J-CTO score; lesions defined as “easy” (J-CTO score 0) or with intermediate difficulty (J-CTO score 1) were excluded from the analysis and only complex cases, defined as those with J-CTO score ≥2 (difficult or very difficult)23, were evaluated. Exclusion criteria comprised contraindications to antiplatelet medication, maximal vessel diameter >4 mm, female patients with childbearing potential or currently breastfeeding and acute ST-segment elevation myocardial infarction.
Patients were considered suitable for the assessment of success rates after successful wire crossing of the CTO lesions.
Invasive imaging was per operator discretion. After implantation, optical coherence tomography (OCT) was performed in a patient subgroup of 10 patients to assess scaffold expansion and incomplete strut apposition (ISA).
The post-procedural drug regimen included dual antiplatelet therapy for at least 12 months followed by lifelong aspirin.
QUANTITATIVE CORONARY ANGIOGRAPHY
Quantitative coronary angiography (QCA) analyses were performed with the Coronary Angiography Analysis System (CAAS; Pie Medical Imaging, Maastricht, the Netherlands). The QCA measurements were performed pre and post BVS implantation. Angiographic views with minimal foreshortening of the lesion and limited overlap with other vessels were used. Pre-procedure QCA analysis was performed as proximally as possible to the occlusion (in case of a side branch, distally to the most proximal take-off of the side branch), as already described24. For each lesion, the following QCA parameters were measured in diastole: minimal lumen diameter (MLD), reference vessel diameter (RVD), percent diameter stenosis (%DS), and occlusion length. The J-CTO score was calculated to estimate lesion complexity and probability of successful guidewire crossing within 30 minutes.
OCT IMAGE ACQUISITION AND ANALYSIS
The C7 system or the ILUMIEN™ OPTIS™ system and the corresponding Dragonfly™ or Dragonfly™ Duo imaging catheters (St. Jude Medical, St. Paul, MN, USA) were used for image acquisition. The OCT catheter was advanced distal to the treated segment, and an automated pullback was performed at 20 mm/s with simultaneous contrast injection at a rate of 3 to 4 ml/s using a power injector. Two sequential pullbacks were performed to enable assessment of the entire scaffolded/stented segment when required.
The OCT measurements were performed offline using the QCU-CMS software (Medis medical imaging systems, Leiden, the Netherlands). Analysis was performed at 1 mm intervals within the entire scaffolded segment and 5 mm proximal and distal to the stent edge. Lumen and scaffold area and diameter measurements were performed in the region of interest (ROI), as appropriate, using standard methodology for the analysis of bioresorbable scaffolds24,25. Eccentricity index and symmetry index were additionally calculated as previously reported26,27.
STUDY DEFINITIONS
Device success was defined as successful BVS implantation with the attainment of ≤30% final in-segment residual stenosis by angiographic visual estimation after Absorb BVS implantation. Procedure success was defined as device success and no major periprocedural complications (emergent CABG, coronary perforation requiring pericardial drainage, residual dissection impairing vessel flow – TIMI flow 2 or less). Clinical success was defined as procedural success and no in-hospital major adverse cardiac events (MACE). All deaths were considered cardiac unless an undisputed non-cardiac cause was identified. Myocardial infarction (MI) and scaffold thrombosis were defined according to the Academic Research Consortium (ARC) definitions28. Any target lesion revascularisation (TLR) was defined as clinically driven if at repeat angiography a diameter stenosis >70% was observed, or if a diameter stenosis >50% was present in association with recurrent angina pectoris, objective signs of ischaemia (ECG changes) at rest or during exercise test likely to be related to the target vessel, or if there were abnormal results of any invasive functional diagnostic test.
MACE was defined as the composite of cardiac death, any reinfarction (Q- or non-Q-wave), emergent bypass surgery (CABG), or clinically driven target lesion revascularisation (TLR).
STATISTICAL ANALYSIS
Categorical variables are reported as counts and percentages and continuous variables as mean±standard deviation. Statistical analyses were performed using SPSS for Windows, Version 20 (IBM Corp., Armonk, NY, USA).
Results
PATIENT DEMOGRAPHICS
Baseline demographics of the patients (N=105) are presented in Table 1. Mean age was 59.40±8.96 years, 89.5% of the patients were male, 33.3% had diabetes mellitus, and 68.6% presented with stable angina.
ANGIOGRAPHIC AND LESION CHARACTERISTICS
The most frequently treated vessel was the right coronary artery (44.8%), 35.2% of the CTO were located at the proximal/ostial coronary segment, 28.6% of the cases involved a bifurcation lesion with a side branch ≥2 mm, 56.2% of the cases showed multivessel disease, 74.3% of the lesions had an occlusion length ≥20 mm, and 43.8% had bridging collaterals at the level of the occlusion. Rentrop grade 3 collaterals were present in 29.5% of the lesions.
The mean J-CTO score was 2.61, with nearly half of the cases (47.6%) having a score ≥3 (Table 2). QCA showed a pre-treatment reference vessel diameter of 2.71±0.55 mm, a post-PCI mean MLD of 2.50±0.51 mm and a percentage diameter stenosis of 14.53±10.31% (Table 2).
PROCEDURAL CHARACTERISTICS
Procedural characteristics are tabulated in Table 3. The majority of the cases (62.9%) were performed with a bifemoral approach and contralateral injections. A retrograde strategy occurred in 25.7% of the procedures (Figure 1). Predilatation was performed in every lesion with a mean predilatation balloon diameter of 2.73±0.43 mm. Guidewires used for successful crossing included the Fielder XT-R/FC (23.8%), Conquest Pro 9/12 (12.4%), Gaia First/Second/Third (11.4%), MIRACLEbros 3/6 (8.6%) (all Asahi Intecc, Aichi, Japan) and Pilot® 150/200 (19.0%) (Abbott Vascular/Guidant, Santa Clara, CA, USA). A total of 256 BVS were implanted with a mean number of scaffolds implanted per lesion of 2.44±1.12, including 79 overlaps, a total scaffold length per lesion of 59.75±25.85 mm and a mean scaffold diameter per lesion of 3.00±0.31 mm. Post-dilatation was performed in 89.5% of the cases.
OCT MEASUREMENTS POST SCAFFOLD DEPLOYMENT
Post scaffold deployment, imaging of the scaffolds with OCT was performed in 10 cases. Mean lumen area was 7.31±1.28 mm2, with a minimum lumen area of 5.35±1.53 mm2 and residual area stenosis of 6.83±26.44%. Mean ISA area was 0.03±0.04 mm2. Mean eccentricity index was 0.86±0.04 and symmetry index was 0.37±0.10 (Table 4).
CLINICAL OUTCOMES
Device success and procedural success were 98.1% and 97.1%, respectively. No severe in-hospital adverse events were observed. The six-month follow-up was completed for 96 patients (91.4%). A total of three events were reported, one periprocedural myocardial infarction, one late scaffold thrombosis, and one additional target lesion revascularisation (Table 5, Figure 2).
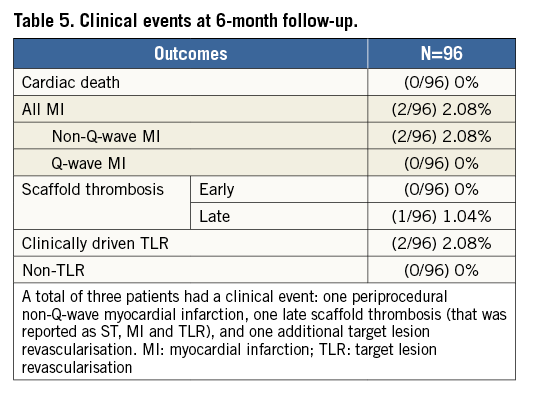
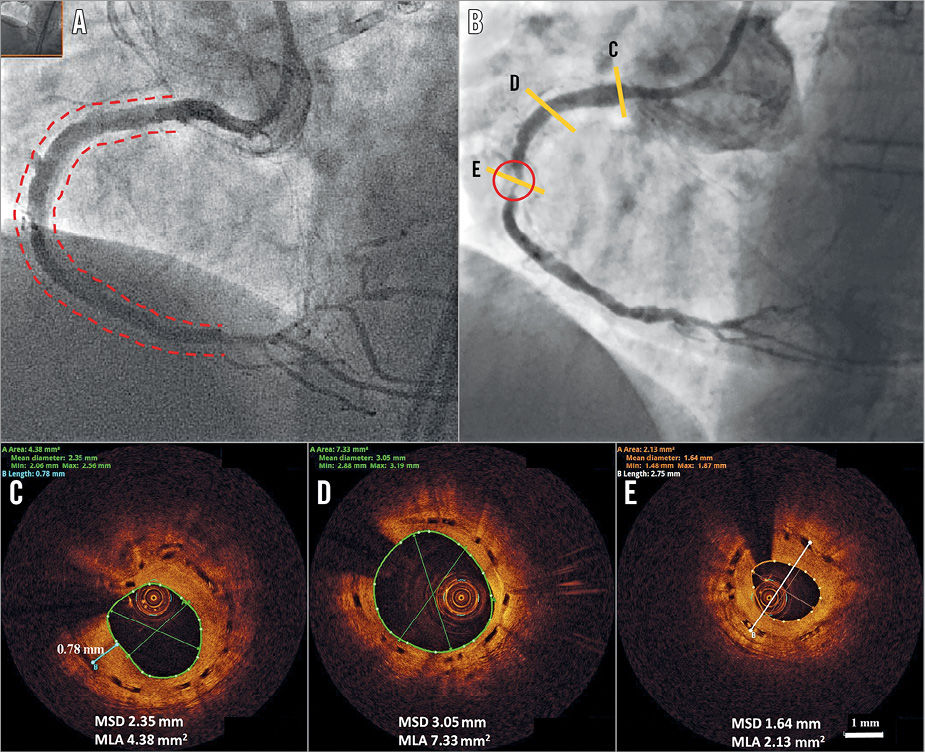
Figure 2. Subintimal BVS implantation. The final result at the index procedure after subintimal tracking, true lumen re-entry and subintimal BVS implantation (A). Two-year angiographic appearance (B) with an in-scaffold restenosis (circled area). C) In-scaffold restenosis. D) & E) Long-term vascular healing after BVS subintimal implantation. MLA: mean lumen area; MSD: mean scaffold diameter
In particular, the patient who developed late scaffold thrombosis had three Absorb BVS scaffolds (3.0×28 mm; 3.5×18 mm and 3.5×18 mm in an overlapping manner) implanted in the left anterior descending artery (J-CTO score 3). The lesion was predilated with a 3.0 mm balloon and post-dilated with a 3.5 mm non-compliant balloon. On day 47 after the index procedure, the patient was re-admitted with a non-ST-segment elevation myocardial infarction while on DAPT. The coronary angiogram showed scaffold thrombosis involving the target vessel which was treated with re-PCI (thrombectomy, implantation of a metal drug-eluting stent and intravenous infusion of eptifibatide).
Computed tomography scans were performed at six months in 34 patients (Figure 3), and revealed scaffold restenosis in two patients. One patient was symptomatic and underwent target lesion revascularisation; the other patient was asymptomatic without inducible ischaemia and was managed conservatively. All patients were alive at six-month follow-up.
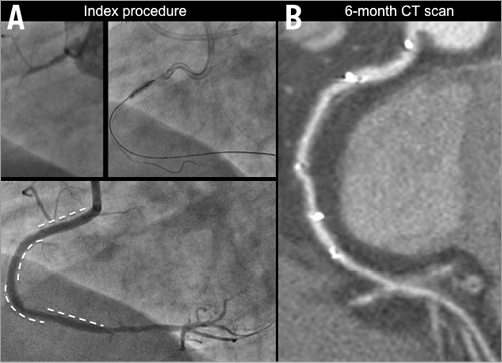
Figure 3. Computed tomography scans at six-month follow-up. An occluded right coronary artery treated with multiple BVS implantation at the index procedure (A). At six-month follow-up the vessel appears patent on CT scan angiography (B). The position of the implanted BVS is described by the presence of the radiopaque markers.
Discussion
Coronary chronic total occlusions are typically regarded as a challenging subset burdened by low procedural success and poorer clinical outcomes when compared to other lesion types. Even though the introduction of drug-eluting stents has reduced the rate of in-stent restenosis, the adoption of these devices is not devoid of limitations, especially in chronically occluded vessels.
Treatment of CTO often implies stenting of long coronary segments with permanent vessel caging of a large part of the artery. This is not only associated with a possible increased risk of restenosis and stent thrombosis, but also precludes future surgical revascularisations in patients who often show diffuse and multivessel atherosclerosis, as is confirmed in the present report with 56.2% of the patients having multivessel disease.
Moreover, a flow-dependent positive vascular remodelling process marks a progressive increase in lumen diameter, external elastic membrane (EEM) diameter, lumen area and EEM area29-31. Park and colleagues29 reported that this increase in lumen dimensions results in more incomplete stent apposition at six-month follow-up in patients treated for CTO lesions. Theoretically, the bioresorption process could eliminate this limitation, as the scaffold remnants can already follow the vessel wall motion between six months and one year after implantation19.
Recently, small series of BVS in CTO reported similar clinical outcomes but they had overall lower lesion complexity20-22. The present investigation focused specifically on complex CTO, illustrated by the fact that half of the CTO were very difficult and we excluded all easy or intermediately difficult CTO as per the J-CTO score. The final angiographic results showed a post-PCI minimal lumen diameter of 2.50±0.51 mm with a low percentage diameter stenosis (14.53±10.31%) and good final TIMI flow. Such promising acute results are in line with angiographic data obtained in previous reports evaluating the BVS as well as metallic DES implantation in CTO lesions20,22,32.
In our series, advanced BVS implantation techniques were applied, including balloon predilatation with a balloon-to-vessel ratio approaching 1:1, scaffold implantation at nominal pressures and post-dilatation with non-compliant balloons up to 5 mm larger than the nominal scaffold diameter. This approach translated into optimal scaffold apposition and expansion with a very low ISA area by OCT. The mean scaffold eccentricity index and symmetry index were similar to those typically reported for BVS and for metal DES in non-chronically occluded vessels26,27,33.
Clinical follow-up at six months demonstrated survival and target lesion revascularisation rates comparable to metal stents in CTO lesions34.
A current limitation of bioresorbable technology is the high strut thickness that could be associated with a delayed vascular healing and endothelialisation, especially at the site of the overlap. In addition, the larger profile of this device compared with previous-generation metallic DES could represent an important obstacle when approaching tortuous, calcified vessels. New delivery systems and reduction in strut thickness and crossing profile may further improve device performance in the future.
Our results support the feasibility of BVS implantation in CTO lesions even in complex scenarios, given an appropriate implantation technique, and justify further research including direct comparison with standard metal drug-eluting stents and analysis of angiographic, intravascular imaging and clinical results at long-term follow-up.
Limitations
This was a single-arm retrospective study. As such, procedural and clinical outcomes cannot be directly compared with lesions treated with metallic drug-eluting stents. Given the limited number of patients and the low rate of events, clinical outcome data should be considered as purely descriptive and hypothesis-generating. The BVS was used as per operator discretion; this methodology may be a source of selection bias. Although personnel trained in an independent core lab performed the QCA analysis, a certified core lab analysis was not available. Invasive imaging was also performed per operator discretion and lacked uniformity.
Conclusions
The present report suggests the feasibility of BVS implantation in complex CTO, given adequate lesion preparation and post-dilatation, with good acute angiographic results and midterm clinical outcomes.
| Impact on daily practice Our observations suggest the feasibility of bioresorbable scaffold implantation in patients with chronic total occlusion and complex anatomies. Optimal antiplatelet regimen, accurate comparison with the current-generation metallic DES and long-term performance remain to be evaluated. |
Guest Editor
This paper was guest edited by Holger Thiele, MD; University Heart Center Lübeck, Medical Clinic II, Lübeck, Germany.
Conflict of interest statement
The authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.

